
|
Developed by 

|
Supported by 

|
Bedaquiline long-acting
Developer(s)

|
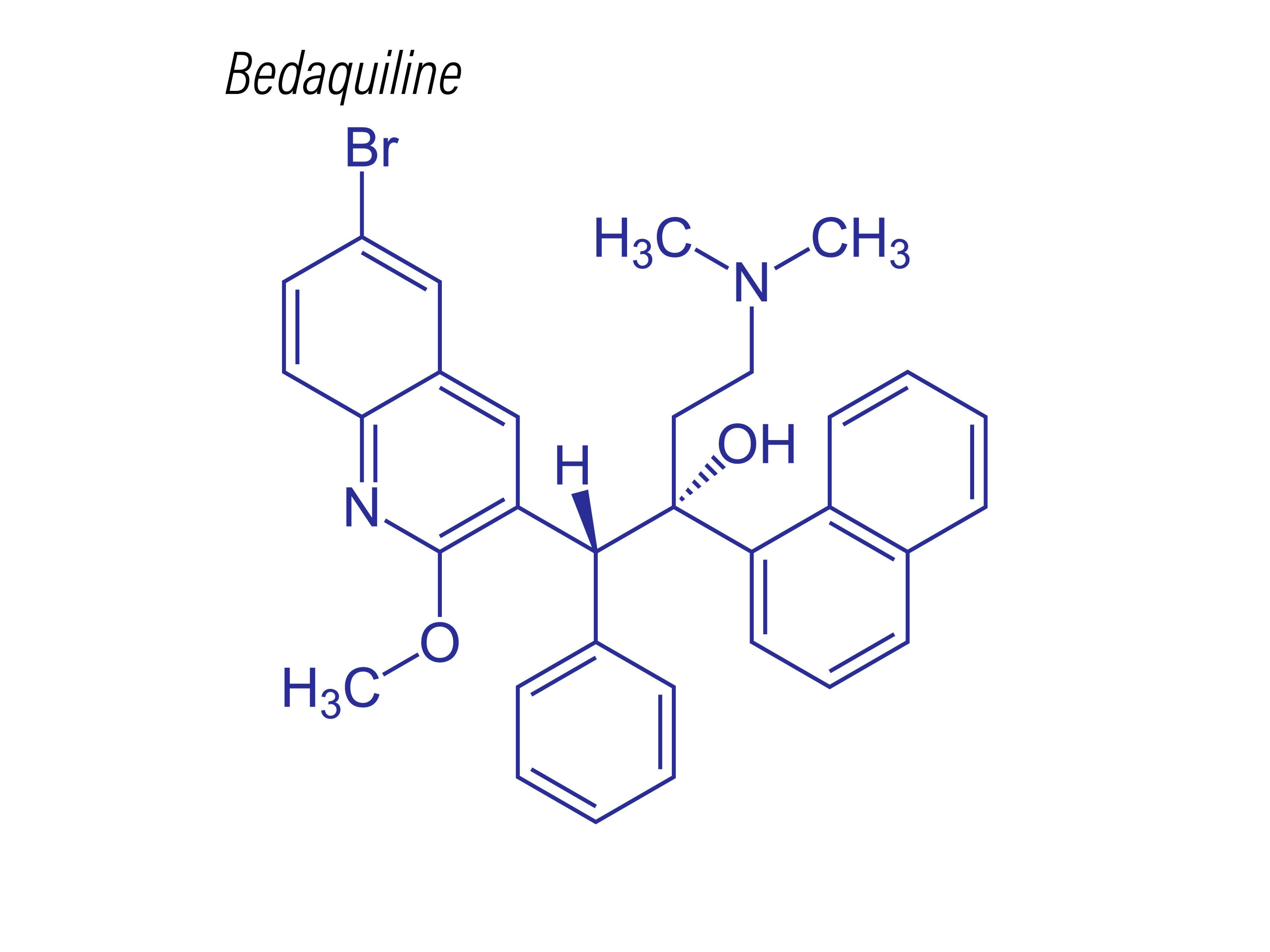
Drug structure

Bedaquiline structure
pubchem
Drug information
Associated long-acting platforms
unknown, unknown
Administration route
Subcutaneous, Intramuscular
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
User acceptance
Not provided
Dosage
Available dose and strength
investigational
Frequency of administration
unknown
Maximum dose
Not provided
Recommended dosing regimen
Not provided
Additional comments
Not provided
Dosage link(s)
Not provided
Drug information
Drug's link(s)
Generic name
Brand name
Compound type
Summary
Approval status
Regulatory authorities
Delivery device(s)
Not provided
Scale-up and manufacturing prospects
Scale-up prospects
Not provided
Tentative equipment list for manufacturing
Not provided
Manufacturing
Not provided
Specific analytical instrument required for characterization of formulation
Not provided
Clinical trials
A Single Ascending Dose, Single-Centre Study, to Assess Pharmacokinetics, Safety and Tolerability of a Single Intramuscular Dose of Bedaquiline Long-Acting Injection Formulation in Healthy Participants
Identifier
EUCT 2023-508810-41-00
Link
https://euclinicaltrials.eu/ctis-public/view/2023-508810-41-00?lang=en
Phase
Phase I
Status
Recruiting
Sponsor
Janssen Cilag International
More details
Not provided
Purpose
Safety and Tolerability of a Single Intramuscular Dose of Bedaquiline Long-Acting Injection Formulation
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2024-06-03 00:00:00
Actual Start Date
2024-07-02 00:00:00
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
Not provided
Estimated Completion Date
2026-02-17 00:00:00
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
study protocol code: TMC207TBC1006
Health status
Study type
Interventional (clinical trial)
Enrollment
Not provided
Allocation
Non-randomized
Intervention model
Not provided
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
Excipients
Proprietary excipients used
Not provided
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
Not provided
Residual solvents used
Not provided
Patent info
Compound patent families
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Bedaquiline Long-acting formulations (suspension of micro- or nanoparticles)
Expiry date: 2038-07-13 This invention concerns pharmaceutical compositions for administration via intramuscular or subcutaneous injection, comprising micro- or nanoparticles of the anti- TB compound bedaquiline, suspended in an aqueous pharmaceutically acceptable carrier, and the use of such pharmaceutical compositions in the treatment and prophylaxis of a pathogenic mycobacterial infection. |
WO2019012100 | Composition | Janssen Pharmaceutica Nv | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | China, Kazakhstan, Morocco, Tunisia, Albania, Serbia, Bosnia and Herzegovina, Cambodia, Montenegro, Türkiye, Moldova, Republic of, North Macedonia, Jordan, Peru, Ukraine, South Africa, Sierra Leone, Eswatini, Liberia, Namibia, Sao Tome and Principe, Mozambique, Uganda, Zambia, Zimbabwe, Tanzania, United Republic of, Malawi, Ghana, Sudan, Botswana, Lesotho, Kenya, Gambia (the), Indonesia, Mexico, Nigeria, Congo, Mauritania, Guinea-Bissau, Niger, Senegal, Cameroon, Mali, Togo, Burkina Faso, Benin, Côte d'Ivoire, Central African Republic, Comoros, Guinea, Gabon, Equatorial Guinea, Chad, Viet Nam | Australia, Russian Federation, Liechtenstein, Italy, Norway, Malta, Denmark, Belgium, United Kingdom, Greece, Netherlands, Hungary, Croatia, Switzerland, Spain, San Marino, Slovenia, Austria, Romania, Iceland, Cyprus, Finland, France, Bulgaria, Slovakia, Poland, Latvia, Ireland, Estonia, Germany, Luxembourg, Portugal, Czechia, Lithuania, Monaco, Sweden, Japan, Korea, Republic of, Saudi Arabia, United States of America, Hong Kong |
| Filed | Brazil, China, Albania, Serbia, Türkiye, North Macedonia, Philippines, Papua New Guinea, Thailand, Uzbekistan | Canada, Liechtenstein, Italy, Norway, Malta, Denmark, Belgium, United Kingdom, Greece, Netherlands, Hungary, Croatia, Switzerland, Spain, San Marino, Slovenia, Austria, Romania, Iceland, Cyprus, Finland, France, Bulgaria, Slovakia, Poland, Latvia, Ireland, Estonia, Germany, Luxembourg, Portugal, Czechia, Lithuania, Monaco, Sweden, Kuwait, Qatar |
| Not in force | World Intellectual Property Organization (WIPO), Colombia, Tajikistan, Belarus, Azerbaijan, Turkmenistan, Armenia, Kyrgyzstan, Morocco, Tunisia, Bosnia and Herzegovina, Cambodia, Montenegro, Moldova, Republic of, India, Rwanda | World Intellectual Property Organization (WIPO), Korea, Republic of |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Bedaquiline fumarate salt and solid compositions
Expiry date: 2027-12-03 Bedaquiline fumarate salt, pharmaceutical compositions comprising as active ingredient said salt and to processes for their preparation. |
WO2008068231 | Salt | Aelterman, Wim, Albert, Alex, Faure, Anne, Hegyi, Jean Francois, Alexandre, Lucas, Janssen Pharmaceutica N.V, Lang, Yolande, Lydia, Leys, Carina, Stokbroekx, Sigrid, Carl, Maria, Van Remoortere, Peter, Jozef, Maria | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Türkiye, Botswana, Gambia (the), Ghana, Kenya, Lesotho, Malawi, Mozambique, Namibia, Sierra Leone, Sudan, Eswatini, Tanzania, United Republic of, Uganda, Zambia, Zimbabwe, Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Moldova, Republic of, Mexico, Peru, South Africa, Lebanon, Indonesia, Jordan, Montenegro, Philippines, Viet Nam, Kosovo, Sri Lanka, Benin, Cameroon, Burkina Faso, Chad, Guinea-Bissau, Mali, Senegal, Congo, Guinea, Gabon, Niger, Equatorial Guinea, Mauritania, Togo, Côte d'Ivoire, Central African Republic, Serbia, North Macedonia, Bosnia and Herzegovina, Albania | United States of America, Australia, Canada, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Slovenia, Hungary, Romania, Poland, Iceland, Lithuania, Latvia, Malta, Hong Kong, Japan, Korea, Republic of, Norway, New Zealand, Taiwan, Province of China, Chile, Russian Federation, Uruguay, Bahrain, Kuwait, Qatar, Saudi Arabia, Oman, United Arab Emirates, Israel, Brunei Darussalam, Panama, Singapore, Croatia |
| Filed | Venezuela (Bolivarian Republic of), Pakistan | |
| Not in force | Argentina, Brazil, China, Malaysia, India, World Intellectual Property Organization (WIPO), Ukraine, Thailand, Egypt | Japan, World Intellectual Property Organization (WIPO) |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Bedaquiline to treat latent TB
Expiry date: 2025-12-08 Use of bedaquiline for the manufacture of a medicament for the treatment of latent tuberculosis |
WO2006067048 | Use | Janssen Pharmaceutica N.V | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Botswana, Gambia (the), Ghana, Kenya, Lesotho, Malawi, Mozambique, Namibia, Sierra Leone, Sudan, Eswatini, Tanzania, United Republic of, Uganda, Zambia, Zimbabwe, Brazil, China, Jordan, Ukraine, South Africa, Montenegro, Indonesia, Sri Lanka, Mexico, Benin, Cameroon, Burkina Faso, Chad, Guinea-Bissau, Mali, Senegal, Congo, Guinea, Gabon, Niger, Equatorial Guinea, Mauritania, Togo, Côte d'Ivoire, Central African Republic, Pakistan, Albania, Bosnia and Herzegovina, North Macedonia | Canada, Australia, Bulgaria, Cyprus, Germany, Denmark, Belgium, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Monaco, Portugal, Ireland, Finland, Czechia, Estonia, Slovakia, Slovenia, Hungary, Romania, Poland, Iceland, Lithuania, Latvia, Hong Kong, Croatia, Israel, Japan, Korea, Republic of, Norway, New Zealand, Taiwan, Province of China, Panama, Bahrain, Kuwait, Qatar, Saudi Arabia, Oman, United Arab Emirates, Macao, Malta, Singapore, Trinidad and Tobago |
| Filed | Nicaragua, Kosovo, Lebanon, Thailand, Venezuela (Bolivarian Republic of) | Cyprus, Germany, Denmark, Spain, Portugal, Slovenia, Poland, Japan |
| Not in force | Türkiye, Argentina, China, Costa Rica, Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Moldova, Republic of, Malaysia, Serbia, India, World Intellectual Property Organization (WIPO), Ecuador, Egypt, Philippines, Viet Nam | Bulgaria, Estonia, Latvia, Japan, Korea, Republic of, United States of America, Russian Federation, World Intellectual Property Organization (WIPO) |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Bedaquiline to treat MDR TB and/or combinations with other antimycobacterial agents
Expiry date: 2025-05-24 The invention relates to the use of a substituted quinoline derivative for the preparation of a medicament for the treatment of an infection with a drug resistant Mycobacterium strain wherein the substituted quinoline derivative is a compound according to Formula (Ia) or Formula (Ib) the pharmaceutically acceptable acid or base addition salts thereof, the stereochemically isomeric forms thereof, the tautomeric forms thereof and the N-oxide forms thereof. Also claimed is a composition comprising a pharmaceutically acceptable carrier and, as active ingredient, a therapeutically effective amount of the above compounds and one or more other antimycobacterial agents. |
WO2005117875 | Use | Janssen Pharmaceutica N.V | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Türkiye, Brazil, China, Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Moldova, Republic of, Mexico, Malaysia, Serbia, South Africa, Indonesia, Kosovo, Lebanon, Benin, Cameroon, Burkina Faso, Chad, Guinea-Bissau, Mali, Senegal, Congo, Guinea, Gabon, Niger, Equatorial Guinea, Mauritania, Togo, Côte d'Ivoire, Central African Republic, Philippines, Venezuela (Bolivarian Republic of), Viet Nam, Montenegro, Jordan, Albania, North Macedonia | Canada, Australia, Germany, Belgium, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Czechia, Estonia, Slovakia, Slovenia, Hungary, Romania, Poland, Iceland, Lithuania, Hong Kong, Israel, Japan, Korea, Republic of, Norway, New Zealand, Taiwan, Province of China, Russian Federation, Panama, Bahrain, Kuwait, Qatar, Saudi Arabia, Oman, United Arab Emirates, Croatia, Latvia, Macao |
| Filed | Pakistan, Thailand, Egypt | |
| Not in force | World Intellectual Property Organization (WIPO), Botswana, Gambia (the), Ghana, Kenya, Lesotho, Malawi, Mozambique, Namibia, Sierra Leone, Sudan, Eswatini, Tanzania, United Republic of, Uganda, Zambia, Zimbabwe, Argentina, China, Ukraine, India, Sri Lanka, Bosnia and Herzegovina | Bulgaria, Korea, Republic of, United States of America, World Intellectual Property Organization (WIPO), Chile |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Bedaquiline compounds
Expiry date: 2023-07-18 Novel compounds, in particular substituted quinoline derivatives, having the property of inhibiting growth of mycobacteria and therefore useful for the treatment of mycobacterial diseases, particularly those diseases caused by pathogenic mycobacteria such as Mycobacterium tuberculosis, M. bovis, M. avium and M. marinum. |
WO2004011436 | Compound | Janssen Pharmaceutica N.V | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Montenegro, Sri Lanka | United States of America, Belgium, Germany, France, Luxembourg, Netherlands, United Kingdom, Sweden, Italy, Austria, Greece, Denmark, Finland, Cyprus, Bulgaria, Estonia, Slovakia, Hungary, Romania, Russian Federation, Israel, Iceland, Japan, Korea, Republic of, Norway, Poland, Taiwan, Province of China, Chile, Latvia, Lithuania, Malta, Singapore |
| Filed | Cyprus | |
| Not in force | Türkiye, Argentina, Brazil, China, Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Moldova, Republic of, World Intellectual Property Organization (WIPO), Mexico, Malaysia, Yugoslavia/Serbia and Montenegro, Ukraine, South Africa, India, Bosnia and Herzegovina, Egypt, Indonesia, Kosovo, Benin, Cameroon, Burkina Faso, Chad, Guinea-Bissau, Mali, Senegal, Congo, Guinea, Gabon, Niger, Equatorial Guinea, Mauritania, Togo, Côte d'Ivoire, Central African Republic, Pakistan, Philippines, Thailand, Viet Nam, Botswana, Gambia (the), Ghana, Kenya, Lesotho, Malawi, Mozambique, Sierra Leone, Sudan, Eswatini, Tanzania, United Republic of, Uganda, Zambia, Zimbabwe, Albania, North Macedonia | Australia, Canada, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Slovenia, Hungary, Romania, Hong Kong, Croatia, New Zealand, World Intellectual Property Organization (WIPO), Bahrain, Kuwait, Qatar, Saudi Arabia, Oman, United Arab Emirates, Latvia, Lithuania |
Supporting material
Publications
Bedaquiline: what might the future hold? Shaw, Emily S et al., The Lancet Microbe, Volume 5, Issue 12, 100909
Tuberculosis drug development has stagnated for decades, so the recent availability of bedaquiline is welcome. Bedaquiline-containing regimens, now the first-line therapy recommended by WHO, have transformed the treatment of drug-resistant tuberculosis, offering safer and more effective oral treatment options. However, key obstacles need to be overcome to ensure global access and prevent the rapid development of resistance against this promising class of drugs. In this Personal View, building on an international workshop held in 2023, we evaluate the current evidence and suggest possible ways forward, recognising the tension between increasing use and slowing the rise of resistance. We also discuss problems in accessing bedaquiline-containing regimens, the potential widening of their use beyond drug-resistant tuberculosis, and lessons for utilising new drugs as they are developed.
Rationale: Completion of preventive therapy is a major bottleneck in global tuberculosis control. Long-acting injectable drug formulations would shorten therapy administration and may thereby improve completion rates. Recently, a long-acting formulation of bedaquiline demonstrated antituberculosis activity for up to 12 weeks after injection in a validated mouse model of preventive therapy. Objectives: The objectives of this study were to 1) determine the total duration of activity after an injection of long-acting bedaquiline and 2) evaluate the activity of regimens comprised of long-acting bedaquiline plus short (2-4 wk) oral companion courses of bedaquiline, with or without rifapentine, using the validated mouse model of tuberculosis preventive therapy. Methods: After the establishment of a stable Mycobacterium tuberculosis lung infection in bacillus Calmette-Guérin (BCG)-immunized BALB/c mice, treatment was initiated with 1 of 12 randomly assigned regimens. In addition to positive and negative controls, six regimens included one or two injections of long-acting bedaquiline (alone or with oral bedaquiline with or without rifapentine), and four comparator regimens consisted of oral agents only. Lung bacterial burden was measured monthly for up to 28 weeks. Measurements and Main Results: One injection of long-acting bedaquiline at 160 mg/kg exerted antituberculosis activity for 12 weeks. Compared with the positive control (daily isoniazid-rifapentine for 4 wk), six regimens had equivalent bactericidal activity (including two all-oral comparator regimens), and two regimens had superior sterilizing activity: one injection with 2 weeks of oral bedaquiline and high-dose rifapentine; and two injections with 4 weeks of oral bedaquiline. Conclusions: Long-acting injectable bedaquiline has significant potential for shortening tuberculosis preventive therapy.
A key component of global tuberculosis (TB) control is the treatment of latent TB infection. The use of long-acting technologies to administer TB preventive treatment has the potential to significantly improve the delivery and impact of this important public health intervention. For example, an ideal long-acting treatment could consist of a single dose that could be administered in the clinic (ie, a “1-shot cure” for latent TB). Interest in long-acting formulations for TB preventive therapy has gained considerable traction in recent years. This article presents an overview of the specific considerations and current preclinical advancements relevant for the development of long-acting technologies of TB drugs for treatment of latent infection, including attributes of target product profiles, suitability of drugs for long-acting formulations, ongoing research efforts, and translation to clinical studies.
The potent antituberculosis activity and long half-life of bedaquiline make it an attractive candidate for use in long-acting/extended-release formulations for the treatment of latent tuberculosis infection (LTBI). Our objective was to evaluate a long-acting injectable (LAI) bedaquiline formulation in a validated paucibacillary mouse model of LTBI.
Additional documents
Useful links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Not provided