
|
Developed by 

|
Supported by 

|
Cabotegravir and Lenacapavir
Developer(s)

|
ViiV Healthcare Originator
https://viivhealthcare.com/
United Kingdom ViiV Healthcare is a pharmaceutical company that specializes in the development of therapies for HIV infection. The company is headquartered in Brentford in the United Kingdom and was initially formed in November 2009 as a part of a joint venture between GlaxoSmithKline and Pfizer. |

|
Gilead Sciences, Inc. Originator
https://www.gilead.com/
United States Gilead Sciences, Inc. is a multinational biopharmaceutical company that develops and manufactures innovative medicines for life-threatening diseases, including anti-viral therapeutics for HIV/AIDS, Hepatitis B, Hepatitis C and Covid-19. Headquartered in Foster City, California, Gilead was originally founded in 1987 and is currently listed on both the S&P 500 and the NASDAQ Biotechnology Index. |
Drug structure
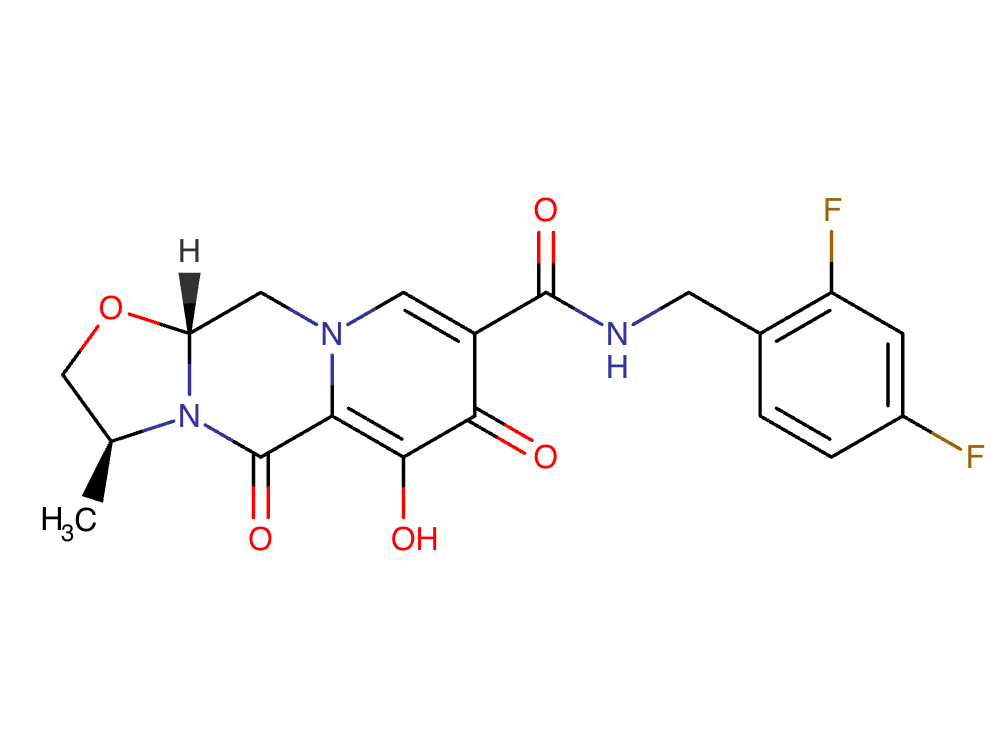
Cabotegravir Chemical Structure
Sourced From DrugBank
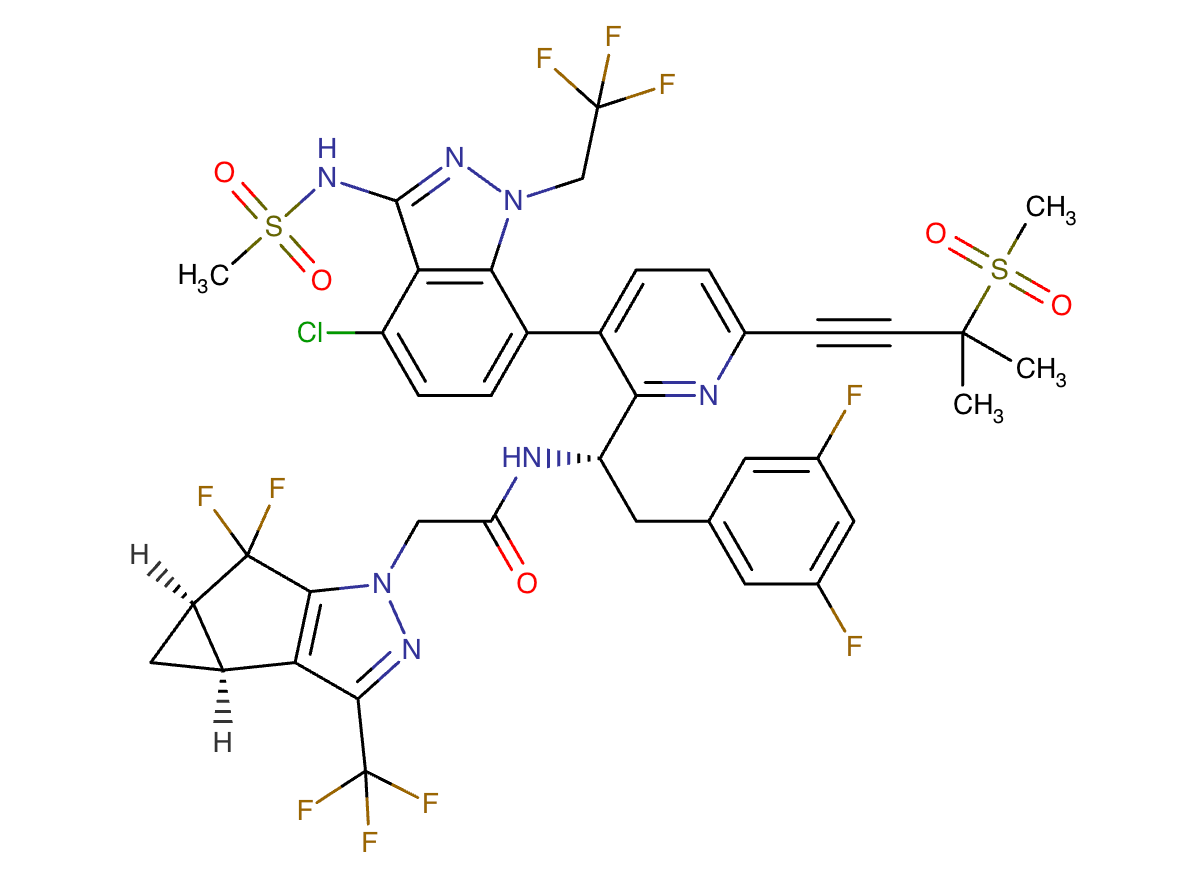
Lenacapavir Chemical Structure
Sourced From DrugBank

Cabotegravir and Lenacapavir Chemical Structure
Composite adapted from individual chemical structures sourced from DrugBank
Drug information
Associated long-acting platforms
Aqueous drug particle suspension, Aqueous solution
Administration route
Subcutaneous, Intramuscular
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
User acceptance
Not provided
Dosage
Available dose and strength
Not provided
Frequency of administration
Not provided
Maximum dose
Not provided
Recommended dosing regimen
Not provided
Additional comments
Not provided
Dosage link(s)
Not provided
Drug information
Generic name
Brand name
Compound type
Summary
Approval status
Regulatory authorities
Delivery device(s)
No delivery device
Scale-up and manufacturing prospects
Scale-up prospects
Cabotegravir is commercially manufactured by the innovator (ViiV Healthcare) and three generic manufacturers have received a licence through the Medicines Patent Pool to manufacture generic versions by 2026/2027. Lenacapavir is commercially manufactured by Gilead Sciences Inc.
Tentative equipment list for manufacturing
Cabotegravir: Conventional wet-bead milling (ball mill), depyrogenated glass vials. Lenacapavir: Equipment: Stainless steel pharmaceutical reactors, glass-lined reactors, rotary evaporator (rotovap), flash chromatography columns, stainless steel autoclave, cooling bath, silica gel chromatography columns, vacuum distillation apparatus, simulated moving bed chromatography system, Chiralpak columns.
Manufacturing
Cabotegravir is subject to a gamma-irradiation pre-sterilization step prior to a conventional wet-bead milling manufacturing procedure. The Cabotegravir milling process is initiated alongside pharmaceutical excipients (polyethylene glycol 3350, water for injection, polysorbate 20 and mannitol) for an overall 200nm drug particle size. Storage of injectable lenacapavir in borosilicate vials is contraindicated due to issues with chemical compatibility. Instead, it is recommended that vials are made from aluminosilicate glass.
Specific analytical instrument required for characterization of formulation
Cabotegravir: PANalytical X’Pert PRO diffractometer equipped with a theta/theta coupled goniometer (or equivalent x-ray powder diffractor) to determine drug particle size, Mettler TGA/DSC 1 instrument for thermal analysis, HPLC to evaluate drug content, impurities and dissolution, HPLC UV-Vis Detector for drug identification. Lenacapavir: Proton nuclear magnetic resonance (1H NMR), High-performance liquid chromatography (HPLC), Ultra-Performance Liquid Chromatography (UPLC).
Clinical trials
CALENDULA
Identifier
NCT06657885
Link
https://clinicaltrials.gov/study/NCT06657885
Phase
Phase II
Status
Not yet recruiting
Sponsor
Institut de Médecine et d'Epidémiologie Appliquée - Fondation Internationale Léon M'Ba
More details
This study is a Phase II, prospective, single-arm, multicenter, non-randomized pilot study designed to evaluate the antiretroviral efficacy of lenacapavir in combination with cabotegravir injection over 48 weeks of follow-up in participants who meet the study inclusion criteria. Efficacy is defined as the absence of virologic failure at S48. Virologic success is defined as maintaining or achieving CV \< 50 copies/mL without interruption of long-acting dual therapy with cabotegravir/lenacapavir at the end of 48 weeks. The study will be conducted at several sites in France in adults 18 years of age and older. Minors and persons under legal guardianship will not be included in the study. Long-acting treatments are evolving thanks to new "long-acting" molecules. These molecules ensure prolong
Purpose
CAbotégravir LENacapavir DUal Long Acting
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2025-01-15
Actual Start Date
Not provided
Anticipated Date of Last Follow-up
2024-10-23
Estimated Primary Completion Date
2026-07-15
Estimated Completion Date
2026-09-15
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Inclusion: - Age ≥ 18 years - HIV-1 infection - Stable oral antiretroviral treatment for at least 6 months - Multi-treated patients who have received multiple lines of antiretroviral treatment - Undetectable patients with CV \< 50 copies/mL in the last 6 months (a single blip between 50 and 200 copies/mL in the last 6 months is allowed) and eligible to switch to the lenacapavir/cabotegravir strategy on the basis of a collegial decision by clinicians, virologists and pharmacologists following a multidisciplinary meeting due to the presence of resistance mutations, including to NNRTIs, oral drug intolerance or drug-drug interactions - Detectable, virologically uncontrolled HIV viral load ≥ 200 c/mL in the last 12 months who is eligible to switch to the lenacapavir/cabotegravir strategy
Health status
Study type
Interventional (clinical trial)
Enrollment
30
Allocation
Non-randomized
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
223616
Identifier
NCT06970223
Link
https://clinicaltrials.gov/study/NCT06970223
Phase
Phase I
Status
Recruiting
Sponsor
ViiV Healthcare
More details
This study will evaluate the tolerability and acceptability of injection site reactions (ISRs) of two long-acting (LA) injectables. Additional characteristics of the ISRs will be investigated and described as well as safety outcomes.
Purpose
A Study to Investigate if Long Acting Cabotegravir (CAB) and Lenacapavir (LEN) Injections Are Tolerable and Acceptable When Administered to Healthy Adults Without HIV
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2025-04-22
Anticipated Date of Last Follow-up
2025-05-05
Estimated Primary Completion Date
2025-07-30
Estimated Completion Date
2026-07-07
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: Participants are eligible to be included in the study only if all the following criteria apply: 1. At the time of obtaining informed consent, 18 years of age. 2. Body weight 50 kg and BMI within the range 18 to 32 kg/m2 (inclusive). 3. Participants who are overtly healthy as determined by medical evaluation by a responsible and experienced physician, including medical history, physical examination, laboratory tests and cardiac monitoring. 4. A participant with a significant clinical abnormality or laboratory parameter(s) which is/are not specifically listed in the inclusion or exclusion criteria, outside the reference range for the population being studied may be included if the investigator determines and documents that the finding is unlikely to introduce additional
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
60
Allocation
Randomized
Intervention model
Cross-over assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
Excipients
Proprietary excipients used
No proprietary excipient used
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
No novel excipient or existing excipient used
Residual solvents used
No residual solvent used
Patent info
There are either no relevant patents or these were not yet submitted to LAPaL
Supporting material
Publications
Gandhi M, Hill L, Grochowski J, Nelson A, Koss CA, Mayorga-Munoz F, Oskarsson J, Shiels M, Avery A, Bamford L, Baron J, Short WR, Hileman CO. Case Series of People With HIV on the Long-Acting Combination of Lenacapavir and Cabotegravir: Call for a Trial. Open Forum Infect Dis. 2024 Apr 16;11(4):ofae125. DOI: 10.1093/ofid/ofae125. PMID: 38628952; PMCID: PMC11020301.
Background
Injectable cabotegravir (CAB)/rilpivirine (RPV) is the only combination long-acting (LA) antiretroviral regimen approved for HIV. RPV may not be effective among individuals with non-nucleoside reverse transcriptase inhibitor (NNRTI) resistance, which has >10% prevalence in many countries. Lenacapavir (LEN) is an LA capsid inhibitor given every 6 months, but has not been studied in combination with other LA agents.
Methods
We assembled a case series from 4 US academic medical centers where patients with adherence challenges were prescribed LEN subcutaneously every 26 weeks/CAB (+/− RPV) intramuscularly every 4 or 8 weeks. Descriptive statistics, including viral load (VL) outcomes, were summarized.
Results
All patients (n = 34: 76% male; 24% cis/trans female; 41% Black; 38% Latino/a; median age [range], 47 [28–75] years; 29% and 71% on CAB every 4 or 8 weeks) reported challenges adhering to oral ART. The reasons for using LEN/CAB with or without RPV were documented or suspected NNRTI mutations (n = 21, 59%), integrase mutations (n = 5, 15%), high VL (n = 6, 18%), or continued viremia on CAB/RPV alone (n = 4, 12%). Injection site reactions on LA LEN were reported in 44% (32% grade I, 12% grade 2). All patients but 2 (32/34; 94%) were suppressed (VL <75 copies/mL) after starting LEN at a median (range) of 8 (4–16) weeks, with 16/34 (47%) suppressed at baseline.
Conclusions
In this case series of 34 patients on LEN/CAB, high rates of virologic suppression (94%) were observed. Reasons for using LEN/CAB included adherence challenges and underlying resistance, mostly to NNRTIs. These data support a clinical trial of LEN/CAB among persons with NNRTI resistance.
Additional documents
No documents were uploaded
Useful links
There are no additional links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Not provided