
|
Developed by 

|
Supported by 

|
Cabotegravir and Rilpivirine
Developer(s)

|
ViiV Healthcare Originator
https://viivhealthcare.com/
United Kingdom ViiV Healthcare is a pharmaceutical company that specializes in the development of therapies for HIV infection. The company is headquartered in Brentford in the United Kingdom and was initially formed in November 2009 as a part of a joint venture between GlaxoSmithKline and Pfizer. |

|
Janssen Pharmaceuticals Originator
https://www.janssen.com/
Belgium Janssen Pharmaceuticals is a subsidiary company of Johnson & Johnson headquartered in Beerse, Belgium. They manufacture and develop pharmaceutical products for use in areas such as, Immunology, Infectious Diseases & Vaccines, Pulmonary Hypertension, Cardiovascular & Metabolism, Oncology, and Neuroscience. |
|
|
ViiV Healthcare(Vocabria) / Janssen-Cilag Ltd (Rekambys) Originator
https://www.janssen.com/ https://viivhealthcare.com/
|
Drug structure
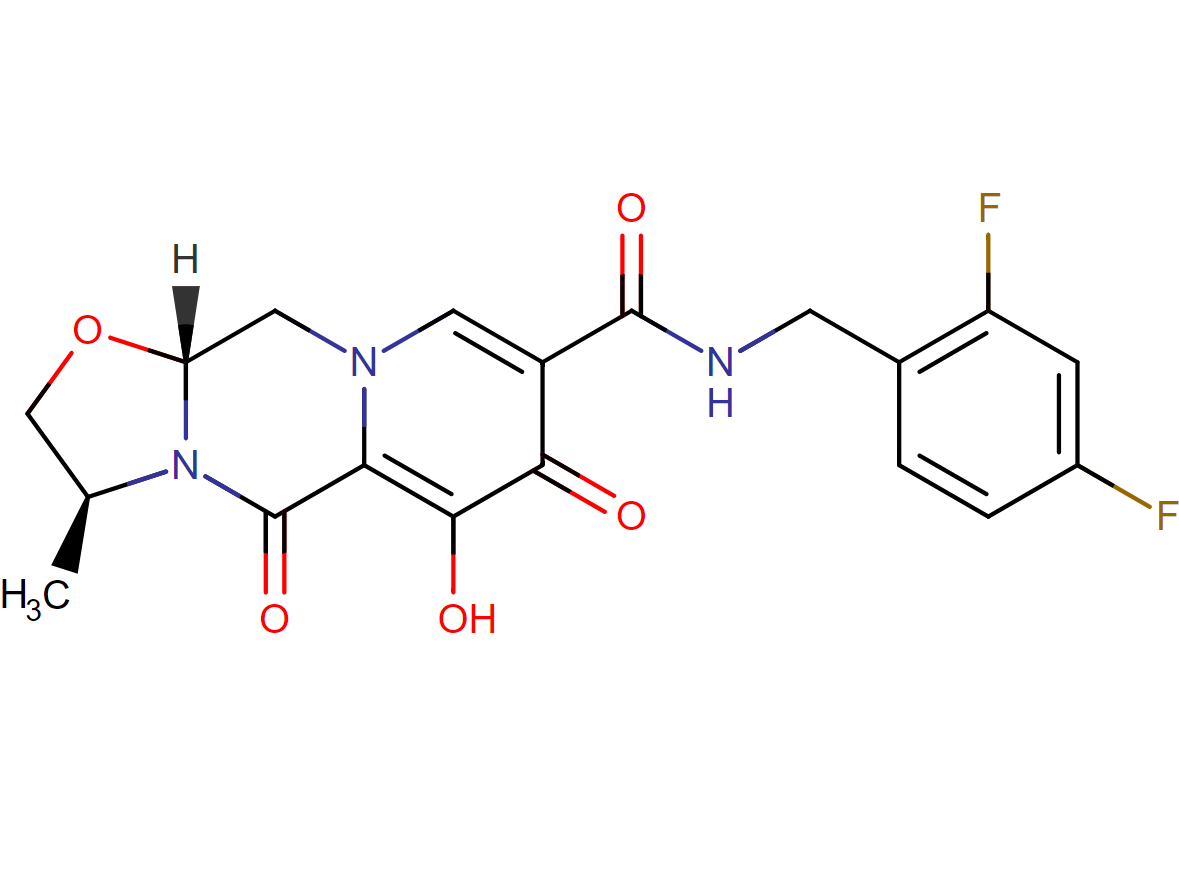
Cabotegravir Chemical Structure
Sourced from DrugBank
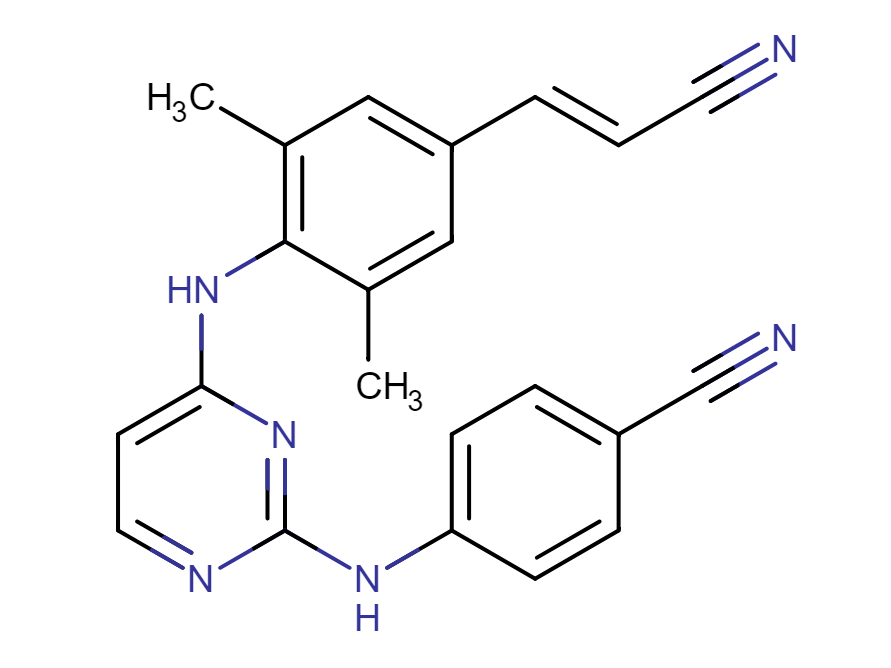
Rilpivirine Chemical Structure
Sourced from DrugBank

CAB/RPV Chemical Structures
Constituent Images Sourced from DrugBank
Drug information
Associated long-acting platforms
Aqueous drug particle suspension
Administration route
Oral, Subcutaneous, Intramuscular
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
User acceptance
Not provided
Dosage
Available dose and strength
Not provided
Frequency of administration
Not provided
Maximum dose
Not provided
Recommended dosing regimen
Not provided
Additional comments
Not provided
Dosage link(s)
Not provided
Drug information
Generic name
Brand name
Compound type
Summary
Approval status
Regulatory authorities
Delivery device(s)
No delivery device
Scale-up and manufacturing prospects
Scale-up prospects
Compounds are commercially manufactured.
Tentative equipment list for manufacturing
Conventional wet-bead milling apparatus (e.g. Netzsch ball mill), depyrogenated glass vials, high pressure homogenizer.
Manufacturing
Cabotegravir and Rilpivirine are formulated into a wet-mill suspension of approximately 200mg/ml and 300mg/ml respectively, due to their low aqueous solubility. This formulation results in the creation of nanocrystal drug particles which are amenable for intramuscular gluteal depot injection. The manufacturing process for RPV is considered to be non-standard due to the inclusion of an aseptic processing step. RPV is light-sensitive, and exposure to light can induce conversion into a Z-isomer form which can affect pharmacokinetic data and activity.
Specific analytical instrument required for characterization of formulation
PANalytical X’Pert PRO diffractometer equipped with a theta/theta coupled goniometer (or equivalent x-ray powder diffractor), Mettler TGA/DSC 1 instrument for thermal analysis, Laser diffractor (determine particle size), FT-IR UHPLC (chemical identification), UHPLC (chromatographic purity), paddle apparatus & UPLC/UV (determine in-vitro drug release for QC / dissolution testing).
Clinical trials
POLAR
Identifier
NCT03639311
Link
https://clinicaltrials.gov/study/NCT03639311
Phase
Phase II
Status
Completed
Sponsor
ViiV Healthcare
More details
Not provided
Purpose
Assess the antiviral activity and safety of CAB LA plus RPV LA, administered Q2M, in approximately 100 adult HIV-1 infected, antiretroviral therapy (ART) experienced participants.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2018-09-24
Anticipated Date of Last Follow-up
2024-05-13
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2019-12-11
Actual Completion Date
2023-01-30
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Participants will rollover from the NCT01641809 (LATTE) study, who have completed minimum duration of Week 312 and with demonstrated HIV-1 ribonucleic acid (RNA) suppression (less than [<]50 copies (c) per milliliter [mL]), while receiving a two-drug regimen consisting of once-daily oral CAB at 30 milligram (mg) plus RPV at 25 mg. The participants will be offered the option to switch to the LA, intramuscular injections of CAB LA plus RPV LA, Q2M or the oral fixed dose combination (FDC) of dolutegravir (DTG) plus RPV, for the continued maintenance of HIV-1 RNA suppression, known as the Maintenance Phase (From Day 1 to Commercial Approval).
Health status
Study type
Interventional (clinical trial)
Enrollment
97
Allocation
Non-randomized
Intervention model
Parallel Assignment
Intervention model description
This is an Intervention Model, with parallel assignment, where the primary purpose of the study is, treatment, with 2 arms and no masking.
Masking
Open label
Masking description
This is an open-label study, thus no masking.
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
| Type | Title | Content | Link |
|---|---|---|---|
| Link | Long-acting cabotegravir and rilpivirine for HIV-1 suppression: switch to 2-monthly dosing after 5 years of daily oral therapy | https://doi.org/10.1097/qad.0000000000003085 |
CUSTOMIZE
Identifier
NCT04001803
Link
https://clinicaltrials.gov/study/NCT04001803
Phase
Phase III
Status
Completed
Sponsor
ViiV Healthcare
More details
Not provided
Purpose
Identify and Evaluate Strategies for Successful Implementation of the Cabotegravir + Rilpivirine Long-acting Injectable Regimen in the US.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-07-08
Anticipated Date of Last Follow-up
2023-03-16
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2020-10-05
Actual Completion Date
2022-03-18
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: - Aged 18 years or older at the time of signing the informed consent. - HIV-1 infected and must be on an active highly active antiretroviral therapy (HAART) (2 or 3 drug) regimen for at least 6 months prior to Screening. - Be able to understand and comply with protocol requirements, instructions, and restrictions. - Understand the long-term commitment to the study and be likely to complete the study as planned. - Be considered appropriate candidates for participation in an investigative clinical trial with oral and intramuscularly injectable medications.
Health status
Study type
Interventional (clinical trial)
Enrollment
115
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
| Type | Title | Content | Link |
|---|---|---|---|
| Link | Perspectives of people living with HIV-1 on implementation of long-acting cabotegravir plus rilpivirine in US healthcare settings | https://doi.org/10.1002/jia2.26006 | |
| Link | Perspectives of healthcare providers on implementation of long-acting cabotegravir plus rilpivirine in US healthcare settings from a Hybrid III Implementation-effectiveness study (CUSTOMIZE) | https://doi.org/10.1002/jia2.26003 |
CR109089
Identifier
NCT05112939
Link
https://clinicaltrials.gov/study/NCT05112939
Phase
Phase I
Status
Completed
Sponsor
Janssen Research & Development, LLC
More details
Not provided
Purpose
Characterize the single dose pharmacokinetics and evaluate the safety and tolerability of subcutaneous administration of RPV LA in combination with CAB LA in different conditions in healthy adults.
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-11-16
Anticipated Date of Last Follow-up
2025-02-27
Estimated Primary Completion Date
2024-05-23
Estimated Completion Date
2024-05-23
Actual Primary Completion Date
2024-05-23
Actual Completion Date
2024-05-23
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Participant must be healthy on the basis of physical examination, clinical laboratory tests, medical history, vital signs, and 12-lead electrocardiogram (ECG).
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
126
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Single blind masking
Masking description
Single (Participant)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
ATLAS-2M
Identifier
NCT03299049
Link
https://clinicaltrials.gov/study/NCT03299049
Phase
Phase III
Status
Not provided
Sponsor
ViiV Healthcare
More details
Not provided
Purpose
Evaluating the Efficacy, Safety, and Tolerability of Long-acting Cabotegravir Plus Long-acting Rilpivirine in HIV-1-infected Adults Who Are Virologically Suppressed.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2017-10-27
Anticipated Date of Last Follow-up
2024-12-10
Estimated Primary Completion Date
Not provided
Estimated Completion Date
2025-12-31
Actual Primary Completion Date
2019-06-06
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: - Subjects who will be able to understand and comply with protocol requirements, instructions, and restrictions. - Understand the long term commitment to the study and be likely to complete the study as planned. - Be considered as an appropriate candidate for participation in an investigative clinical trial with oral and intramuscularly injectable medications (e.g., no active substance use disorder, acute major organ disease, or planned long-term work assignments out of the country, etc.). - Aged 18 years or older (or >=19 where required by local regulatory agencies), at the time of signing the informed consent.
Health status
Study type
Interventional (clinical trial)
Enrollment
1049
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Two groups of subjects will be randomized to receive CAB LA + RPV LA Q4W, or CAB LA + RPV LA Q8W regimen.
Masking
Open label
Masking description
This will be an open-label study and therefore no blinding is required.
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
| Type | Title | Content | Link |
|---|---|---|---|
| Link | Indirect comparison of 48-week efficacy and safety of long-acting cabotegravir and rilpivirine maintenance every 8 weeks with daily oral standard of care antiretroviral therapy in participants | https://doi.org/10.1186/s12879-022-07243-3 | |
| Link | Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 96-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study | https://doi.org/10.1016/s2352-3018(21)00185-5 | |
| Link | Week 96 extension results of a Phase 3 study evaluating long-acting cabotegravir with rilpivirine for HIV-1 treatment | https://doi.org/10.1097/qad.0000000000003025 | |
| Link | Patient-Reported Outcomes Through 1 Year of an HIV-1 Clinical Trial Evaluating Long-Acting Cabotegravir and Rilpivirine Administered Every 4 or 8 Weeks (ATLAS-2M) | https://doi.org/10.1007/s40271-021-00524-0 | |
| Link | Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 48-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study | https://doi.org/10.1016/s0140-6736(20)32666-0 |
SOLAR
Identifier
NCT04542070
Link
https://clinicaltrials.gov/study/NCT04542070
Phase
Phase III
Status
Completed
Sponsor
ViiV Healthcare
More details
Not provided
Purpose
Assess the antiviral activity and safety of a two-drug regimen of CAB LA + RPV LA compared with maintenance of BIK. BIKTARVY is a registered trademark of Gilead Sciences.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2020-11-09
Anticipated Date of Last Follow-up
2024-06-03
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-07-13
Actual Completion Date
2023-04-17
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: - Participants aged 18 years or older (or >=19 where required by local regulatory agencies), at the time of signing the informed consent. - A female participant is eligible to participate if she is not pregnant (as confirmed by a negative serum human chorionic gonadotropin (hCG) test at screen and a negative urine hCG test at Randomization). - Must be on the uninterrupted current regimen of BIK for at least 6 months prior to Screening with an undetectable HIV-1 viral load for at least 6 months prior to Screening. BIK must be the participant's first or second regimen. - Capable of giving signed informed consent, which includes compliance with the requirements and restrictions listed in the consent form and in this protocol.
Health status
Study type
Interventional (clinical trial)
Enrollment
687
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
| Type | Title | Content | Link |
|---|---|---|---|
| Link | Factors Associated with Health Care Providers' Preference for Forgoing an Oral Lead-In Phase When Initiating Long-Acting Injectable Cabotegravir and Rilpivirine in the SOLAR Clinical Trial | https://doi.org/10.1089/apc.2022.0168 |
ATLAS
Identifier
NCT02951052
Link
https://clinicaltrials.gov/study/NCT02951052
Phase
Phase III
Status
Not provided
Sponsor
ViiV Healthcare
More details
Not provided
Purpose
Establish if HIV-1 infected adult subjects with current viral suppression on a regimen with 2 NRTIs plus a third agent, remain suppressed upon switching to a 2 drug intramuscular regime of CAB/RPV-LA.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2016-10-28
Anticipated Date of Last Follow-up
2025-01-09
Estimated Primary Completion Date
Not provided
Estimated Completion Date
2025-12-31
Actual Primary Completion Date
2018-05-29
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Must be on uninterrupted current ARV regimen (either the initial or second ARV regimen) for at least 6 months prior to Screening. Any prior switch, defined as a change of a single drug or multiple drugs simultaneously, must have occurred due to tolerability/safety, access to medications, or convenience/simplification, and must NOT have been done for treatment failure (HIV-1 RNA ≥400 c/mL).
Health status
Study type
Interventional (clinical trial)
Enrollment
618
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
| Type | Title | Content | Link |
|---|---|---|---|
| Link | Indirect comparison of 48-week efficacy and safety of long-acting cabotegravir and rilpivirine maintenance every 8 weeks with daily oral standard of care antiretroviral therapy in participants | https://doi.org/10.1186/s12879-022-07243-3 | |
| Link | Week 96 extension results of a Phase 3 study evaluating long-acting cabotegravir with rilpivirine for HIV-1 treatment | https://doi.org/10.1097/qad.0000000000003025 | |
| Link | Long-Acting Injectable Cabotegravir + Rilpivirine for HIV Maintenance Therapy: Week 48 Pooled Analysis of Phase 3 ATLAS and FLAIR Trials | https://doi.org/10.1097/qai.0000000000002466 | |
| Link | Long-Acting Cabotegravir and Rilpivirine for Maintenance of HIV-1 Suppression | https://doi.org/10.1056/nejmoa1904398 |
FLAIR
Identifier
NCT02938520
Link
https://clinicaltrials.gov/study/NCT02938520
Phase
Phase III
Status
Not provided
Sponsor
ViiV Healthcare
More details
Not provided
Purpose
Establish if HIV-1 infected adult participants whose virus is virologically suppressed on an INI STR will remain suppressed after switching to a two drug LA regimen of CAB and RPV.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2016-10-27
Anticipated Date of Last Follow-up
2025-03-04
Estimated Primary Completion Date
Not provided
Estimated Completion Date
2025-12-31
Actual Primary Completion Date
2018-08-30
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Antiretroviral-naive (<=10 days of prior therapy with any antiretroviral agent following a diagnosis of HIV-1 infection). Any previous exposure to an HIV integrase inhibitor or non-nucleoside reverse transcriptase inhibitor will be exclusionary.
Health status
Study type
Interventional (clinical trial)
Enrollment
631
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
| Type | Title | Content | Link |
|---|---|---|---|
| Link | Indirect comparison of 48-week efficacy and safety of long-acting cabotegravir and rilpivirine maintenance every 8 weeks with daily oral standard of care antiretroviral therapy in participants | https://doi.org/10.1186/s12879-022-07243-3 | |
| Link | Long-Acting Injectable Cabotegravir + Rilpivirine for HIV Maintenance Therapy: Week 48 Pooled Analysis of Phase 3 ATLAS and FLAIR Trials | https://doi.org/10.1097/qai.0000000000002466 | |
| Link | Long-Acting Cabotegravir and Rilpivirine after Oral Induction for HIV-1 Infection | https://doi.org/10.1056/nejmoa1909512 | |
| Link | Impact of Integrase Sequences from HIV-1 Subtypes A6/A1 on the In Vitro Potency of Cabotegravir or Rilpivirine | https://doi.org/10.1128/aac.01702-21 | |
| Link | Initiation of long-acting cabotegravir plus rilpivirine as direct-to-injection or with an oral lead-in in adults with HIV-1 infection | https://doi.org/10.1016/s2352-3018(21)00184-3 | |
| Link | Long-acting cabotegravir plus rilpivirine for treatment in adults with HIV-1 infection: 96-week results of the randomised, open-label, phase 3 FLAIR study | https://doi.org/10.1016/s2352-3018(20)30340-4 |
CARISEL
Identifier
NCT04399551
Link
https://clinicaltrials.gov/study/NCT04399551
Phase
Phase III
Status
Completed
Sponsor
ViiV Healthcare
More details
Not provided
Purpose
Evaluating Implementation Strategies for Cabotegravir (CAB)+ Rilpivirine (RPV) Long-acting (LA) Injectables for Human Immunodeficiency Virus (HIV)-1 Treatment in European Countries
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2020-09-28
Anticipated Date of Last Follow-up
2024-04-04
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-03-07
Actual Completion Date
2023-03-13
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
HIV-1 infected and must be suppressed on a guideline recommended active Highly active antiretroviral therapy (HAART) regimen for at least 6 months prior to screening. Any prior switch, defined as a change of a single drug or multiple drugs simultaneously, must have occurred due to tolerability/safety, access to medications, or convenience/simplification, and must not have been done for virologic failure (on treatment HIV-1 RNA more than or equal to [>=]200 c/mL).
Health status
Study type
Interventional (clinical trial)
Enrollment
437
Allocation
Non-randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
This is an open-label study hence no blinding is required.
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
| Type | Title | Content | Link |
|---|---|---|---|
| Link | Top Practices for Implementing Cabotegravir (CAB) and Rilpivirine (RPV) Long-Acting (LA) in European Clinics | https://www.bhiva.org/file/62a1ceca806ef/P008.pdf |
LATA
Identifier
NCT05154747
Link
https://clinicaltrials.gov/study/NCT05154747
Phase
Phase III
Status
Not provided
Sponsor
University College, London
More details
Not provided
Purpose
Comparing the efficacy of long-acting injectable CAB+RPV administered every two months in comparison to daily oral HIV medications in young people.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-06-22
Anticipated Date of Last Follow-up
2024-04-26
Estimated Primary Completion Date
2025-03-01
Estimated Completion Date
2026-03-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Children
- Adolescents
- Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Study participants are individuals with HIV-1 infection aged 12-19 years in Sub-Saharan Africa. Participants with known HIV-2 infection are excluded.
Health status
Study type
Interventional (clinical trial)
Enrollment
476
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
IMPALA
Identifier
NCT05546242
Link
https://clinicaltrials.gov/study/NCT05546242
Phase
Phase III
Status
Not provided
Sponsor
MRC/UVRI and LSHTM Uganda Research Unit
More details
Not provided
Purpose
Evaluating the Effectiveness of Switching to Two-monthly Long-acting Injectable CAB and RPV From First-line Oral Antiretroviral Therapy in HIV-1 Positive Virologically Suppressed Adults in SSA.
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-12-08
Anticipated Date of Last Follow-up
2024-09-25
Estimated Primary Completion Date
2025-04-01
Estimated Completion Date
2026-03-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Participants must have a history of sub-optimal ART adherence or engagement in care based on one or more of the following criteria: 1. Documented detectable HIV-1 VL (>1000 c/mL) on all-oral ART (EFV/NVP or DTG-based) in the prior 2 years despite being ART-experienced for ≥3 months. 2. History of being lost to follow-up from care (>4 weeks elapsed since a missed scheduled clinic appointment or refill in the prior 2 years). 3. Failed to link to HIV care despite ≥3 months elapsed since HIV diagnosis.
Health status
Study type
Interventional (clinical trial)
Enrollment
540
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Parallel open-label phase 3b study. Participants will be randomised to continuing current therapy or switching to injectable therapy.
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
LATITUDE
Identifier
NCT03635788
Link
https://clinicaltrials.gov/study/NCT03635788
Phase
Phase III
Status
Not provided
Sponsor
National Institute of Allergy and Infectious Diseases (NIAID)
More details
Not provided
Purpose
Compare the efficacy, safety, and durability of two different strategies to treat participants with a history of sub-optimal adherence and control of their HIV infection.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-03-28
Anticipated Date of Last Follow-up
2024-11-19
Estimated Primary Completion Date
2024-09-30
Estimated Completion Date
2026-08-30
Actual Primary Completion Date
2024-10-15
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Evidence of non-adherence to ART according to at least one of the following criteria: 1. Poor virologic response within 18 months prior to study entry (defined as less than 1 log10 decrease in HIV-1 RNA or HIV-1 RNA greater than 200 copies/mL at two time points at least 4 weeks apart) in individuals who have been prescribed ART for at least 6 consecutive months. 2. Lost to clinical follow-up within 18 months prior to study entry with ART non-adherence for greater than or equal to 6 consecutive months.
Health status
Study type
Interventional (clinical trial)
Enrollment
310
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
LATTE-2
Identifier
NCT02120352
Link
https://clinicaltrials.gov/study/NCT02120352
Phase
Phase II
Status
Completed
Sponsor
ViiV Healthcare
More details
Not provided
Purpose
Evaluate the antiviral activity, tolerability, and safety of IM dosing regimens of GSK744 LA plus TMC278 LA, relative to GSK744 plus ABC/3TC given orally once daily, in ARV naïve HIV-1 patients.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2014-04-28
Anticipated Date of Last Follow-up
2024-06-11
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2015-08-13
Actual Completion Date
2023-04-20
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Participants must be ART-naïve defined as having no more than 10 days of prior therapy with any antiretroviral agent following a diagnosis of HIV-1 infection.
Health status
Study type
Interventional (clinical trial)
Enrollment
309
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
| Type | Title | Content | Link |
|---|---|---|---|
| Link | Experiences with long acting injectable ART: A qualitative study among PLHIV participating in a Phase II study of cabotegravir + rilpivirine (LATTE-2) in the United States and Spain. | https://doi.org/10.1371/journal.pone.0190487 | |
| Link | Efficacy, Safety, and Durability of Long-Acting Cabotegravir and Rilpivirine in Adults With Human Immunodeficiency Virus Type 1 Infection: 5-Year Results From the LATTE-2 Study. | https://doi.org/10.1093/ofid/ofab439 | |
| Link | Pharmacokinetics and antiviral activity of cabotegravir and rilpivirine in cerebrospinal fluid following long-acting injectable administration in HIV-infected adults. | https://doi.org/10.1093/jac/dkz504 | |
| Link | Patient-reported tolerability and acceptability of cabotegravir + rilpivirine long-acting injections for the treatment of HIV-1 infection: 96-week results from the randomized LATTE-2 study. | https://doi.org/10.1080/25787489.2019.1661696 | |
| Link | Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. | https://doi.org/10.1016/s0140-6736(17)31917-7 |
NCT04371380
Identifier
NCT04371380
Link
https://clinicaltrials.gov/study/NCT04371380
Phase
Phase I
Status
Completed
Sponsor
ViiV Healthcare
More details
Not provided
Purpose
Evaluate pharmacokinetics, tolerability, and safety of Cabotegravir long acting plus Rilpivirine long acting administered concomitantly as two separate IM injections in the Vastus Lateralis muscles.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2020-09-16
Anticipated Date of Last Follow-up
2023-11-03
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2021-12-26
Actual Completion Date
2021-12-26
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Participants aged 18 to 50 who are overtly healthy as determined by medical evaluation including medical history, physical examination, laboratory tests, and cardiac monitoring.
Health status
Study type
Interventional (clinical trial)
Enrollment
15
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Eligible participants will receive orally, tablets of cabotegravir plus rilpivirine for 28 days. There will be 10 to 14 days wash out period followed by an IM injection of cabotegravir long-acting plus rilpivirine long-acting.
Masking
Open label
Masking description
This is an open label study.
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
| Type | Title | Content | Link |
|---|---|---|---|
| Link | Pharmacokinetics and Tolerability of Cabotegravir and Rilpivirine Long-Acting Intramuscular Injections to the Vastus Lateralis (Lateral Thigh) Muscles of Healthy Adult Participants. | https://medinfo.gsk.com/5f95dbd7-245e-4e65-9f36-1a99e28e5bba/75cb786a-98e0-4615-8258-3cae0bdcfb29/75cb786a-98e0-4615-8258-3cae0bdcfb29_viewable_rendition__v.pdf |
LAI115428
Identifier
NCT01593046
Link
https://clinicaltrials.gov/study/NCT01593046
Phase
Phase I
Status
Completed
Sponsor
ViiV Healthcare
More details
Not provided
Purpose
Investigate the Safety, Tolerability and Pharmacokinetics of Repeat Dose Administration of Long-Acting GSK1265744 and Long-Acting TMC278 Intramuscular and Subcutaneous Injections.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2012-05-01
Anticipated Date of Last Follow-up
2014-02-06
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2013-11-01
Actual Completion Date
2013-11-01
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: - AST, ALT, alkaline phosphatase and bilirubin greater than or equal to 1.5xULN (isolated bilirubin >1.5xULN is acceptable if bilirubin is fractionated and direct bilirubin <35%). - Healthy as determined by a responsible and experienced physician. - Male or female between 18 and 64 years of age inclusive, at the time of signing the informed consent. - Body weight greater than or equal to 50 kg for men and greater than or equal to 45 kg for women and body mass index (BMI) within the range 18.5-31.0 kg/m2 (inclusive). - All Study subjects should be counseled on the practice of safer sexual practices including the use of effective barrier methods (e.g. male condom/spermicide).
Health status
Study type
Interventional (clinical trial)
Enrollment
43
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Unspecified
Key resources
CAPRI
Identifier
NCT05601128
Link
https://clinicaltrials.gov/study/NCT05601128
Phase
Phase III
Status
Not provided
Sponsor
Allegheny Singer Research Institute
More details
Not provided
Purpose
Evaluate the efficacy and safety of CABENUVA (Long-acting Cabotegravir Plus Long-acting Rilpivirine) in patients with HIV infection and severe renal impairment.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-01-01
Anticipated Date of Last Follow-up
2025-02-06
Estimated Primary Completion Date
2025-04-01
Estimated Completion Date
2025-12-31
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Participants are positive for HIV infection and severe renal impairment with or without hemodialysis.
Health status
Study type
Interventional (clinical trial)
Enrollment
12
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
MOCHA
Identifier
NCT03497676
Link
https://clinicaltrials.gov/study/NCT03497676
Phase
Phase I/II
Status
Not provided
Sponsor
National Institute of Allergy and Infectious Diseases (NIAID)
More details
Not provided
Purpose
Evaluate the safety, acceptability, tolerability, and pharmacokinetics of oral and long-acting injectable CAB and RPV in virologically suppressed HIV-infected children and adolescents.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-04-03
Anticipated Date of Last Follow-up
2024-09-03
Estimated Primary Completion Date
Not provided
Estimated Completion Date
2025-06-17
Actual Primary Completion Date
2023-02-18
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Children
- Adolescents
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Not provided
Health status
Study type
Interventional (clinical trial)
Enrollment
168
Allocation
Non-randomized
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
| Type | Title | Content | Link |
|---|---|---|---|
| Link | Safety and pharmacokinetics of oral and long-acting injectable cabotegravir or long-acting injectable rilpivirine in virologically suppressed adolescents with HIV (IMPAACT 2017/MOCHA) | https://doi.org/10.1016/s2352-3018(23)00300-4 | |
| Link | Acceptability and tolerability of long-acting injectable cabotegravir or rilpivirine in the first cohort of virologically suppressed adolescents living with HIV (IMPAACT 2017/MOCHA): | https://doi.org/10.1016/s2352-3018(23)00301-6 |
VOLITION
Identifier
NCT05917509
Link
https://clinicaltrials.gov/study/NCT05917509
Phase
Phase III
Status
Not provided
Sponsor
ViiV Healthcare
More details
Not provided
Purpose
Evaluate the efficacy, safety, implementation effectiveness, and patient-reported outcomes of once-daily oral DTG/3TC followed by an optional participant-determined switch to CAB/RPV-LA.
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-07-06
Anticipated Date of Last Follow-up
2025-01-15
Estimated Primary Completion Date
2025-10-02
Estimated Completion Date
2026-10-02
Actual Primary Completion Date
2026-01-30
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Antiretroviral-naïve participants (defined as no prior therapy with any antiretroviral agent following a diagnosis of HIV-1 infection) prior to enrolment with plasma HIV-1 RNA ≥1,000 c/mL at screening. Participants enrolled in France must be affiliated to, or a beneficiary of, a social security category.
Health status
Study type
Interventional (clinical trial)
Enrollment
171
Allocation
Non-randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
ALADDIN
Identifier
NCT06468995
Link
https://clinicaltrials.gov/study/NCT06468995
Phase
Phase III
Status
Recruiting
Sponsor
IRCCS San Raffaele
More details
This is a monocentric, prospective, double-arm, randomized, open-label, implementation-effectiveness hybrid type III study aimed at comparing hospital-based and home-based administration of CAB LA + RPV LA treatment for HIV-1-infected patients. Study participants receiving IM CAB + RPV will complete various questionnaires and scales, including FIM, AIM, IAM, EQ-5D-5L, HAT-QoL, and HIVTSQ, throughout the study. HCPs will also complete FIM, AIM, IAM, and a Likert scale.
Purpose
Antiviral Long Acting Drugs Landing in People Living With HIV
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2024-09-01
Actual Start Date
2024-12-02
Anticipated Date of Last Follow-up
2024-12-04
Estimated Primary Completion Date
2026-03-01
Estimated Completion Date
2026-11-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: - People living with HIV-1 infection that could, according to clinical practice, switch current ART to IM CAB + RPV; - Aged 18 years or older at the time of signing the informed consent. - People willing to switch to long-acting therapy - On a stable (≥6 months) antiretroviral regimen and virologically suppressed (HIV-1 RNA \<50 copies/ml): - Documented evidence of plasma HIV-1 RNA measurements \<50 c/mL in the 6 months prior to Screening. - Plasma HIV-1 RNA \<50 c/mL at Screening. - Ability to understand informed consent form and other relevant regulatory documents.
Health status
Study type
Interventional (clinical trial)
Enrollment
120
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
CROWN
Identifier
NCT06694805
Link
https://clinicaltrials.gov/study/NCT06694805
Phase
Phase III
Status
Recruiting
Sponsor
ViiV Healthcare
More details
This study will assess how effective, safe, and long-lasting a long-acting antiretroviral therapy (ART) using CAB LA + RPV LA is for people with HIV who still have detectable virus levels despite being on oral ART. The study will also consider feedback from patients on their experience with this treatment.
Purpose
A Study to Evaluate the Effectiveness of Long-acting (LA) Cabotegravir (CAB) + Rilpivirine (RPV) LA When Given to Participants With Detectable HIV-1
Interventions
Not provided
Countries
Not provided
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2024-12-02
Anticipated Date of Last Follow-up
2025-02-18
Estimated Primary Completion Date
2026-05-13
Estimated Completion Date
2027-12-08
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Children
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Age 1. Aged \>=12 years and \>=35 kg (at the time of obtaining informed consent). * Type of Participant and Disease Characteristics 2.HIV-1 infection, documented by any licensed rapid HIV test or HIV enzyme or chemiluminescence immunoassay (E/CIA) test kit at any time prior to study entry and confirmed by a licensed Western blot or a second antibody test by a method other than the initial rapid HIV and/or E/CIA, or by HIV-1 antigen, plasma HIV-1 RNA VL. 3.Plasma HIV-1 RNA \>1 000 c/mL and greater than (\<) 100 000 c/mL at Screening. 4.Evidence of insufficient virologic response to participant's current oral ART regimen within 18 months prior to study entry according to at least 1 of the following criteria: i.\<1 log10 decrease in HIV-1 RNA or HIV-1 RNA \>200 c/
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
332
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Not provided
Studied LA-formulation(s)
Not provided
Studied route(s) of administration
Not provided
Use case
Not provided
Key resources
Excipients
Proprietary excipients used
No proprietary excipient used
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
The novel excipient poloxamer 338 (P338) is used in the final G001 Rilpivirine clinical formulation. Following both an in-vitro mammalian chromosome aberration and an Ames test, it was considered to be non-genotoxic with no evidence for mutagenicity. Further P338 fertility, genotoxicity and development studies have been conducted with no negative effects, in addition to a 6-week and 9- month minipig repeat-dose toxicity study. No adverse local or systemic toxicity was reported in the minipigs at 100mg/month (Margin of Exposure:19).
Residual solvents used
No residual solvent used
Patent info
Compound patent families
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Cabotegravir+Rilpivirine HIV intramuscular treatment regimens every 4 weeks (once a month) or 8 weeks (once every two months)
Expiry date: 2038-07-18 |
WO2019016732 | Use | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Mexico | Canada, Japan, Russian Federation, United States of America |
| Filed | Argentina, China, Serbia, Montenegro, Türkiye, North Macedonia | Chile, Liechtenstein, Italy, Norway, Malta, Denmark, Belgium, United Kingdom, Greece, Netherlands, Hungary, Croatia, Switzerland, Spain, San Marino, Slovenia, Austria, Romania, Iceland, Cyprus, Finland, France, Bulgaria, Slovakia, Poland, Latvia, Ireland, Estonia, Germany, Luxembourg, Portugal, Czechia, Lithuania, Monaco, Sweden, Israel, Taiwan, Province of China |
| Not in force | China, Morocco, Tunisia, Albania, Bosnia and Herzegovina, Cambodia, Moldova, Republic of, World Intellectual Property Organization (WIPO) | Australia, Japan, Korea, Republic of, United States of America, World Intellectual Property Organization (WIPO) |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Cabotegravir long-acting parenteral compositions
Expiry date: 2031-09-15 The present invention relates to pharmaceutical compositions of cabotegravir useful in the treatment or prevention of Human Immunodeficiency Virus (HIV) infections. |
WO2012037320 | Composition | Glaxosmithkline Llc, Mundhra, Deepak B, Pan, Rennan, Viiv Healthcare Company | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Brazil, China, Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Moldova, Republic of, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro, Serbia, Mexico, Ukraine, South Africa, India | Australia, Canada, Chile, Russian Federation, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania, Slovenia, Israel, Japan, Korea, Republic of, Taiwan, Province of China, United States of America |
| Filed | United States of America | |
| Not in force | World Intellectual Property Organization (WIPO) | World Intellectual Property Organization (WIPO), United States of America |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Cabotegravir processes and intermediates
Expiry date: 2031-03-22 Relates to the preparation of carbamoylpyridone derivatives and intermediates which are useful as HIV integrase inhibitors. |
WO2011119566 | Intermediate(s), Process | Glaxosmithkline Llc | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | China, Albania, Serbia, Bosnia and Herzegovina, Montenegro, Türkiye, North Macedonia, India | Liechtenstein, Italy, Norway, Malta, Denmark, Belgium, United Kingdom, Greece, Netherlands, Hungary, Croatia, Switzerland, Spain, San Marino, Slovenia, Austria, Romania, Iceland, Cyprus, Finland, France, Bulgaria, Slovakia, Poland, Latvia, Ireland, Estonia, Germany, Luxembourg, Portugal, Czechia, Lithuania, Monaco, Sweden, Japan, Korea, Republic of, United States of America |
| Filed | San Marino, Singapore, Taiwan, Province of China | |
| Not in force | World Intellectual Property Organization (WIPO) | World Intellectual Property Organization (WIPO) |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Cabotegravir in combination with RPV
Expiry date: 2031-01-24 A combination comprising cabotegraviror a pharmaceutically acceptable salt thereof, and one or more therapeutic agents selected from the group consisting of rilpivirine (TMC-278), or a pharmaceutically acceptable salt thereof, when administered simultaneously or sequentially. |
CA3060290 | Combination | Viiv Healthcare Company | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Mexico | New Zealand, Korea, Republic of, Australia, Israel |
| Filed | Canada | |
| Not in force | Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro, Serbia | Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania, Slovenia |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Aqueous suspensions of rilpivirine micro- or nanoparticles
Expiry date: 2027-06-22 This invention concerns pharmaceutical compositions for administration via intramuscular or subcutaneous injection, comprising micro- or nanoparticles of the NNRTI compound TMC278, suspended in an aqueous pharmaceutically acceptable carrier, and the use of such pharmaceutical compositions in the treatment and prophylaxis of HIV infection. |
WO2007147882 | Composition | Tibotec Pharmaceuticals Ltd | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Brazil, China, Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Moldova, Republic of, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Serbia, Mexico, Ukraine, India, Benin, Cameroon, Burkina Faso, Chad, Guinea-Bissau, Mali, Senegal, Congo, Guinea, Gabon, Niger, Equatorial Guinea, Mauritania, Togo, Côte d'Ivoire, Central African Republic, Jordan, Philippines, Thailand | Canada, Australia, Chile, Cyprus, Denmark, Russian Federation, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Monaco, Portugal, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Croatia, Romania, Latvia, Lithuania, Slovenia, Israel, Japan, Korea, Republic of, New Zealand, Singapore, United States of America, Bahrain, Kuwait, Qatar, Saudi Arabia, Oman, United Arab Emirates |
| Filed | Jordan, Pakistan, Venezuela (Bolivarian Republic of) | Cyprus, Denmark, Spain, Portugal, Finland, Hungary, Poland, Croatia, Lithuania, Slovenia, Taiwan, Province of China, Uruguay, Brunei Darussalam, Macao, Hong Kong |
| Not in force | Botswana, Gambia (the), Ghana, Kenya, Lesotho, Malawi, Mozambique, Namibia, Sierra Leone, Sudan, Eswatini, Tanzania, United Republic of, Uganda, Zambia, Zimbabwe, Argentina, Peru, World Intellectual Property Organization (WIPO), Indonesia, Egypt, South Africa, Viet Nam | United States of America, World Intellectual Property Organization (WIPO), Panama |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Rilpivine parenteral formulation
Expiry date: 2027-01-19 This invention relates to the use of a parenteral formulation comprising an anti-virally effective amount of TMC278 or a pharmaceutically acceptable acid-addition salt thereof, and a carrier, for the manufacture of a medicament for the treatment of a subject being infected with HIV, wherein the formulation is to be administered intermittently at a time interval of at least one week. |
WO2007082922 | Dose/Regimen, Use | Tibotec Pharmaceuticals Ltd | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Moldova, Republic of, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Serbia, Montenegro, Malaysia, Ukraine, Philippines, Thailand, Benin, Cameroon, Burkina Faso, Chad, Guinea-Bissau, Mali, Senegal, Congo, Guinea, Gabon, Niger, Equatorial Guinea, Mauritania, Togo, Côte d'Ivoire, Central African Republic, Mexico, Nigeria, South Africa | Canada, Australia, Russian Federation, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Croatia, Romania, Latvia, Lithuania, Slovenia, Hong Kong, Israel, Japan, Korea, Republic of, New Zealand, Singapore, Taiwan, Province of China, United States of America, Malta |
| Filed | Spain, Cyprus, Croatia, Hong Kong, Japan, Korea, Republic of, Singapore, United States of America | |
| Not in force | Botswana, Gambia (the), Ghana, Kenya, Lesotho, Malawi, Mozambique, Namibia, Sierra Leone, Sudan, Eswatini, Tanzania, United Republic of, Uganda, Zambia, Zimbabwe, Argentina, Brazil, China, World Intellectual Property Organization (WIPO), India, Egypt, Viet Nam, Indonesia | United States of America, World Intellectual Property Organization (WIPO), Chile |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Dolutegravir and Cabotegravir compounds
Expiry date: 2026-04-28 The present invention is to provide a novel compound (I), having the anti-virus activity, particularly the HIV integrase inhibitory activity, and a drug containing the same, particularly an anti-HIV drug, as well as a process and an intermediate thereof. Compound (I) wherein Z<1> is NR<4>; R<1> is hydrogen or lower alkyl; X is a single bond, a hetero atom group selected from O, S, SO, SO2 and NH, or lower alkylene or lower alkenylene in which the hetero atom group may intervene; R<2> is optionally substituted aryl; R<3> is hydrogen, a halogen, hydroxy, optionally substituted lower alkyl etc; and R<4> and Z<2> part taken together forms a ring, to form a polycyclic compound, including e.g., a tricyclic or tetracyclic compound. |
WO2006116764 | Compound | Glaxosmithkline Llc | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Brazil, China, Morocco, Mexico, Philippines, Ukraine, Viet Nam, South Africa, Türkiye, Armenia, Azerbaijan, Belarus, Kyrgyzstan, Kazakhstan, Moldova, Republic of, Tajikistan, Turkmenistan, Nigeria, Colombia, Indonesia, Malaysia, Algeria | United States of America, Australia, Canada, Cyprus, Hong Kong, Israel, Japan, Korea, Republic of, Luxembourg, Norway, New Zealand, Taiwan, Province of China, Austria, Belgium, Bulgaria, Switzerland, Czechia, Germany, Denmark, Estonia, Spain, Finland, France, United Kingdom, Greece, Hungary, Ireland, Iceland, Italy, Liechtenstein, Lithuania, Latvia, Monaco, Netherlands, Poland, Portugal, Romania, Sweden, Slovenia, Slovakia, Russian Federation, Trinidad and Tobago, Singapore |
| Filed | Egypt | United States of America, Cyprus, Luxembourg, Norway, Finland, France, Hungary, Lithuania, Netherlands, Slovenia |
| Not in force | Türkiye, India, World Intellectual Property Organization (WIPO) | United States of America, Cyprus, Hong Kong, Israel, Japan, Luxembourg, Austria, Belgium, Bulgaria, Switzerland, Czechia, Germany, Denmark, Estonia, Spain, Finland, France, United Kingdom, Greece, Hungary, Ireland, Iceland, Italy, Liechtenstein, Lithuania, Latvia, Monaco, Netherlands, Poland, Portugal, Romania, Sweden, Slovenia, Slovakia, World Intellectual Property Organization (WIPO) |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Rilpivirine compound and analogues and their use in HIV
Expiry date: 2022-08-09 The present invention is concerned with pyrimidine derivatives having HIV (Human Immunodeficiency Virus) replication inhibiting properties. The invention further relates to methods for their preparation and pharmaceutical compositions comprising them. The invention also relates to the use of said compounds for the manufacture of a medicament for the prevention or the treatment of HIV infection. |
WO03016306 | Compound | Janssen Pharmaceutica N.V | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Brazil, Ukraine, Kazakhstan, Albania | Australia, Germany, Hungary, Israel, Japan, Korea, Republic of, Luxembourg, Norway, Poland, Slovenia, Taiwan, Province of China, United States of America, Austria, Belgium, Bulgaria, Switzerland, Cyprus, Czechia, Denmark, Estonia, Spain, Finland, France, Greece, Ireland, Italy, Liechtenstein, Monaco, Netherlands, Portugal, Sweden, Slovakia, Russian Federation, Chile, Romania, Latvia, Lithuania, Singapore |
| Filed | Venezuela (Bolivarian Republic of) | Hungary, Slovenia, Cyprus, France, Lithuania |
| Not in force | Argentina, Brazil, China, Egypt, Mexico, South Africa, Botswana, Ghana, Gambia (the), Kenya, Lesotho, Malawi, Mozambique, Sudan, Sierra Leone, Eswatini, Tanzania, United Republic of, Uganda, Zambia, Zimbabwe, Türkiye, Armenia, Azerbaijan, Belarus, Kyrgyzstan, Moldova, Republic of, Tajikistan, Turkmenistan, Burkina Faso, Benin, Central African Republic, Congo, Côte d'Ivoire, Cameroon, Gabon, Guinea, Equatorial Guinea, Guinea-Bissau, Mali, Mauritania, Niger, Senegal, Chad, Togo, India, Malaysia, Philippines, Thailand, Viet Nam, Sri Lanka, World Intellectual Property Organization (WIPO), Albania, North Macedonia, Jordan, Lebanon, Pakistan, Indonesia | Canada, Germany, Hong Kong, Croatia, Japan, Luxembourg, Norway, New Zealand, Panama, Poland, Slovenia, Taiwan, Province of China, United States of America, Austria, Belgium, Bulgaria, Switzerland, Cyprus, Czechia, Denmark, Estonia, Spain, Finland, France, United Kingdom, Greece, Ireland, Italy, Liechtenstein, Monaco, Netherlands, Portugal, Sweden, Slovakia, World Intellectual Property Organization (WIPO), Romania, Latvia, Lithuania, Kuwait, United Arab Emirates, Bahrain, Saudi Arabia, Oman, Qatar, Macao, Trinidad and Tobago |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Rilpivirine compound and analogues (Markush structure)
Expiry date: 2021-02-26 Pyrimidine derivatives of formula (I) wherein Q1, Q2, G and R<1> are as defined within; and pharmaceutically acceptable salts and in vivo hydrolysable esters thereof are described. Processes for their manufacture, pharmaceutical compositions and their use as cyclin-dependent serine/threonine kinase (CDK) and focal adhesion kinase (FAK) inhibitors are also described. |
WO0164656 | Compound | Astrazeneca Ab, Astrazeneca Uk Limited | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Austria, Belgium, Denmark, Finland, France, Greece, Ireland, Italy, Luxembourg, Netherlands, Sweden | |
| Filed | Norway, Cyprus, France | |
| Not in force | World Intellectual Property Organization (WIPO), Brazil, China, Mexico, South Africa, Türkiye, Albania, North Macedonia | Canada, United Kingdom, World Intellectual Property Organization (WIPO), Australia, Hong Kong, Israel, Japan, Korea, Republic of, Norway, New Zealand, United States of America, Switzerland, Cyprus, Germany, Spain, Liechtenstein, Monaco, Portugal, Romania, Latvia, Lithuania, Slovenia |
Supporting material
Publications
Bares SH, Scarsi KK. A new paradigm for antiretroviral delivery: long-acting cabotegravir and rilpivirine for the treatment and prevention of HIV. Curr Opin HIV AIDS. 2022 Jan 1;17(1):22-31. doi: https://doi.org/10.1097/COH.0000000000000708. PMID: 34871188; PMCID: PMC8694245.
Purpose of review
Cabotegravir (CAB) and rilpivirine (RPV) is the first long-acting injectable antiretroviral therapy (ART) option approved for virologically suppressed adults with HIV-1. In addition, long-acting CAB is a promising agent for HIV preexposure prophylaxis (PrEP). This review focuses on phase 3 clinical trial results and implementation considerations for these long-acting ART and PrEP strategies.
Recent findings
Long-acting CAB and RPV administered every 4 weeks demonstrated noninferiority to oral ART through week 96 in both the ATLAS and FLAIR studies, whereas ATLAS-2M found similar efficacy through 96 weeks when the long-acting injectable ART was administered every 8 weeks instead of every 4 weeks. For prevention, two phase 3 trials were stopped early due to fewer incident HIV infections in participants receiving long-acting CAB every 8 weeks compared with daily oral tenofovir disoproxil fumarate–emtricitabine for PrEP. The long-acting therapies were well tolerated across all clinical trials.
Summary
Clinical trial results support the use of long-acting CAB for HIV PrEP and long-acting CAB and RPV as a switch strategy for adults with HIV-1 who are first virologically suppressed with oral ART. Implementation challenges persist, and data are urgently needed in populations who may benefit most from long-acting therapy, including adolescents, pregnant individuals, and those with barriers to medication adherence.
Additional documents
No documents were uploaded
Useful links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Not provided