
|
Developed by 

|
Supported by 

|
Cabotegravir (CAB)
Developer(s)

|
ViiV Healthcare Originator
https://viivhealthcare.com/
United Kingdom ViiV Healthcare is a pharmaceutical company that specializes in the development of therapies for HIV infection. The company is headquartered in Brentford in the United Kingdom and was initially formed in November 2009 as a part of a joint venture between GlaxoSmithKline and Pfizer. |
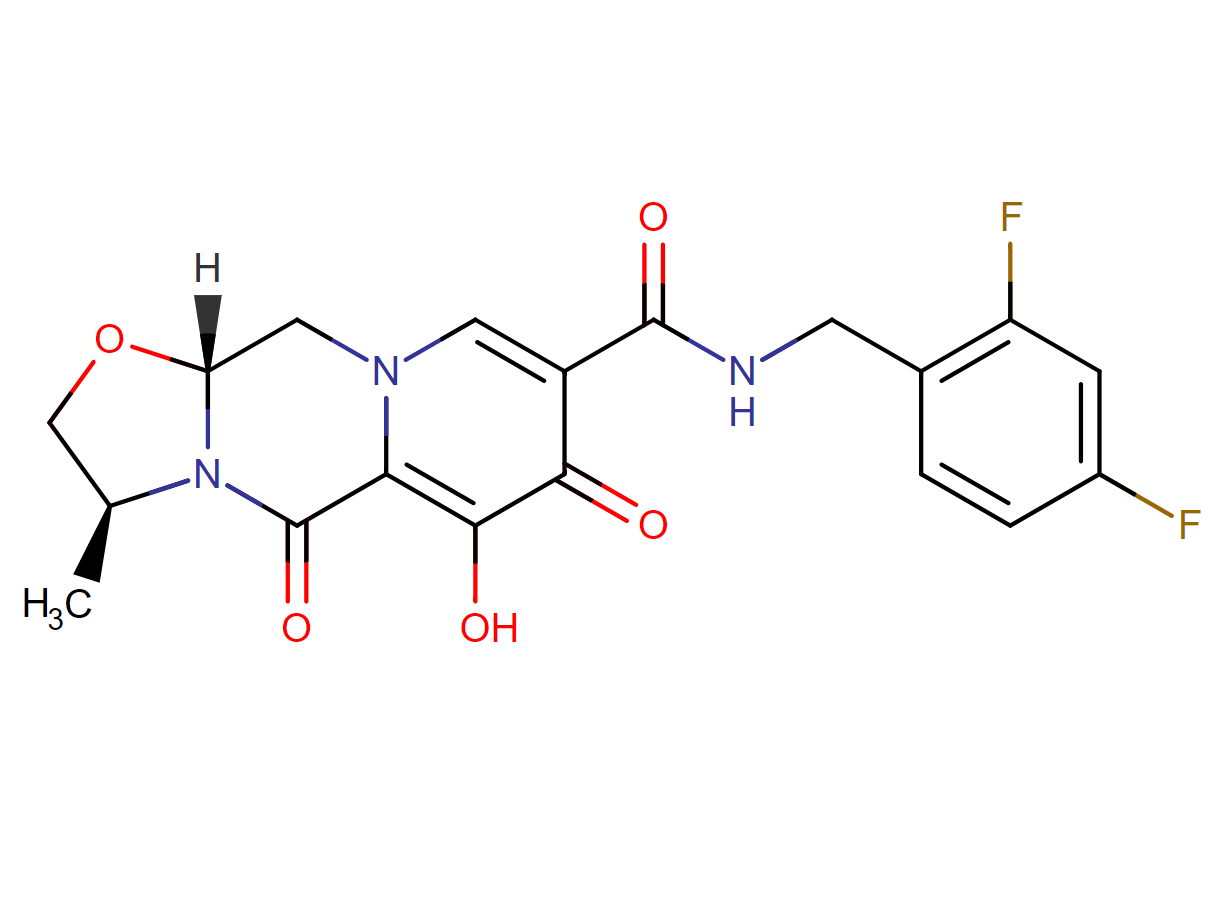
Drug structure

Cabotegravir Chemical Structure
Sourced from DrugBank
Drug information
Associated long-acting platforms
Aqueous drug particle suspension
Administration route
Oral, Intramuscular
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
User acceptance
Not provided
Dosage
Available dose and strength
600 mg/3 mL
Frequency of administration
Once monthly (Q1M); Two months once (Q2M)
Maximum dose
200 mg/mL
Recommended dosing regimen
Not provided
Additional comments
Not provided
Drug information
Drug's link(s)
Not provided
Generic name
Brand name
Compound type
Summary
Approval status
Regulatory authorities
Delivery device(s)
No delivery device
Scale-up and manufacturing prospects
Scale-up prospects
Compound is commercially manufactured by the innovator and three generic manufacturers have received a licence through the medicines patent pool to manufacture generic versions by 2026/2027.
Tentative equipment list for manufacturing
Conventional wet-bead milling (ball mill), depyrogenated glass vials.
Manufacturing
Cabotegravir is subject to a gamma-irradiation pre-sterilization step prior to a conventional wet-bead milling manufacturing procedure. The Cabotegravir milling process is initiated alongside pharmaceutical excipients (polyethylene glycol 3350, water for injection, polysorbate 20 and mannitol) for an overall 200nm drug particle size. Sterilized de-pyrogenated glass vials are used to store the finished drug nanosuspension, before an additional gamma irradiation (25kGy) step to ensure aseptic packaging conditions.
Specific analytical instrument required for characterization of formulation
PANalytical X’Pert PRO diffractometer equipped with a theta/theta coupled goniometer (or equivalent x-ray powder diffractor) to determine drug particle size, Mettler TGA/DSC 1 instrument for thermal analysis, HPLC to evaluate drug content, impurities and dissolution, HPLC UV-Vis Detector for drug identification.
Clinical trials
ECLAIR
Identifier
NCT02076178
Link
https://clinicaltrials.gov/study/NCT02076178
Phase
Phase II
Status
Completed
Sponsor
ViiV Healthcare
More details
Not provided
Purpose
Evaluate the safety, tolerability and acceptability of long acting injections of the HIV integrase inhibitor, GSK1265744, in HIV-uninfected men.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2014-03-27
Anticipated Date of Last Follow-up
2017-12-13
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2015-05-15
Actual Completion Date
2016-02-23
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Cisgender male
- Transgender male
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: - Non-reactive HIV test at screening or enrollment. - Males 18 to 65 years old at the time of signing the informed consent. - At risk of acquiring HIV, defined as having at least one casual sex partner in the past 24 months. - Healthy as determined by a responsible and experienced physician, based on a medical evaluation including medical history, physical examination, laboratory tests and cardiac monitoring at the time of screening. - If participating in sexual activity with a female of child-bearing potential, men must agree to use condoms. Female partner must use contraception. - Capable of giving written informed consent, which includes compliance with the requirements and restrictions listed in the consent form. - Willing to undergo all required study procedures.
Health status
Study type
Interventional (clinical trial)
Enrollment
127
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Double-blind masking
Masking description
Double (Participant, Investigator)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
| Type of key results | Title | Website link |
|---|---|---|
| Article | Satisfaction and acceptability of cabotegravir long-acting injectable suspension for prevention of HIV: Patient perspectives from the ECLAIR trial | https://pubmed.ncbi.nlm.nih.gov/30445896/ |
| Article | Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): a multicentre, double-blind, randomised, placebo-controlled, phase 2a trial | https://pubmed.ncbi.nlm.nih.gov/28546090/ |
HPTN 077
Identifier
NCT02178800
Link
https://clinicaltrials.gov/study/NCT02178800
Phase
Phase II
Status
Completed
Sponsor
National Institute of Allergy and Infectious Diseases
More details
Not provided
Purpose
Evaluate the safety, tolerability, and pharmacokinetics of an investigational, injectable HIV medicine (GSK1265744) in HIV-uninfected adults.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2015-02-01
Anticipated Date of Last Follow-up
2021-10-14
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2017-04-05
Actual Completion Date
2018-07-13
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Men and women, 18 to 65 years old at the time of screening, who are willing to provide informed consent for the study. Participants are required to be in general good health, as confirmed by laboratory investigation, with no medical condition(s) that would interfere with the conduct of the study.
Health status
Study type
Interventional (clinical trial)
Enrollment
199
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
| Type of key results | Title | Website link |
|---|---|---|
| Article | Tail-phase safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected adults: a secondary analysis of the HPTN 077 trial | https://pubmed.ncbi.nlm.nih.gov/32497491/ |
| Article | Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial | https://pubmed.ncbi.nlm.nih.gov/30408115/ |
HPTN 083
Identifier
NCT02720094
Link
https://clinicaltrials.gov/study/NCT02720094
Phase
Phase II/III
Status
Active, not recruiting
Sponsor
National Institute of Allergy and Infectious Diseases
More details
Not provided
Purpose
Evaluate the safety and efficacy of the injectable drug cabotegravir (CAB LA), for pre-exposure prophylaxis (PrEP) in HIV-uninfected cisgender men and transgender women who have sex with men.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2016-12-01
Anticipated Date of Last Follow-up
2024-03-11
Estimated Primary Completion Date
Not provided
Estimated Completion Date
2024-07-01
Actual Primary Completion Date
2020-05-14
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Cisgender male
- Transgender female
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
HIV negative cis-gender men and transgender women (18 years or older at the time of screening [assigned male at birth]) who have sex with men and at high risk of HIV infection.
Health status
Study type
Interventional (clinical trial)
Enrollment
4570
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
| Type of key results | Title | Website link |
|---|---|---|
| Article | Cabotegravir for HIV Prevention in Cisgender Men and Transgender Women | https://pubmed.ncbi.nlm.nih.gov/34379922/ |
HPTN 084
Identifier
NCT03164564
Link
https://clinicaltrials.gov/study/NCT03164564
Phase
Phase III
Status
Active, not recruiting
Sponsor
National Institute of Allergy and Infectious Diseases
More details
Not provided
Purpose
Evaluate the safety and efficacy of CAB LA compared to daily oral tenofovir disoproxil fumarate/emtricitabine for PrEP in HIV-uninfected women.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2017-11-07
Anticipated Date of Last Follow-up
2023-03-21
Estimated Primary Completion Date
Not provided
Estimated Completion Date
2024-11-30
Actual Primary Completion Date
2020-11-05
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
Genders
- Cisgender female
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Born female and 18-45 years of age at the time of screening. Must have documented evidence of surgical sterilization, OR documented evidence of no uterus (e.g., hysterectomy), OR must agree to use a reliable form of long acting contraception, during the trial and for 52 weeks after stopping the long acting injectable, or 30 days after stopping oral study product
Health status
Study type
Interventional (clinical trial)
Enrollment
3224
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
| Type of key results | Title | Website link |
|---|---|---|
| Article | Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial | https://pubmed.ncbi.nlm.nih.gov/35378077/ |
EBONI
Identifier
NCT05514509
Link
https://clinicaltrials.gov/study/NCT05514509
Phase
Marketed
Status
Recruiting
Sponsor
ViiV Healthcare
More details
Not provided
Purpose
Evaluate implementation strategies for the delivery of CAB for HIV PrEP across clinical settings for adult (≥18 Years) black cis-and transgender women without HIV infection living in the United States
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-10-28
Anticipated Date of Last Follow-up
2024-05-30
Estimated Primary Completion Date
2025-09-30
Estimated Completion Date
2025-09-30
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Cisgender female
- Transgender female
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion criteria: - Participant must be ≥18 years of age, at the time of signing the informed consent. - HIV negative at screening. Type of HIV-1 test is per standard of care. - No prior history of receiving oral CAB or CAB LA injections. - PrEP provider deems CAB PrEP use to be appropriate per the applicable CAB PrEP prescribing information prior to enrollment in the study. - Female at birth or self-identified Transgender Female. - Self-identified as African American/Black. - Capable of giving signed informed consent.
Health status
Study type
Interventional (clinical trial)
Enrollment
250
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
PILLAR
Identifier
NCT05374525
Link
https://clinicaltrials.gov/study/NCT05374525
Phase
Marketed
Status
Recruiting
Sponsor
ViiV Healthcare
More details
Not provided
Purpose
Evaluate implementation strategies for the delivery of cabotegravir PrEP for HIV uninfected MSM and transgender men ≥ 18 in the United States.
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-05-18
Anticipated Date of Last Follow-up
2024-02-13
Estimated Primary Completion Date
2024-09-06
Estimated Completion Date
2024-09-06
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Cisgender male
- Transgender male
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Not provided
Health status
Study type
Interventional (clinical trial)
Enrollment
116
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
ImPrEP
Identifier
NCT05515770
Link
https://clinicaltrials.gov/study/NCT05515770
Phase
Phase III
Status
Not yet recruiting
Sponsor
Evandro Chagas National Institute of Infectious Disease
More details
Not provided
Purpose
Assess the safety and effectiveness of open label CAB LA PrEP when offered at public health facilities to cisgender men and transgender or gender non-binary individuals who have sex with men.
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2022-09-20
Actual Start Date
Not provided
Anticipated Date of Last Follow-up
2022-08-24
Estimated Primary Completion Date
2025-10-01
Estimated Completion Date
2026-02-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
Genders
- Cisgender male
- Transgender female
- Transgender male
- Gender non-binary
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Cisgender men, non-binary (assigned as male at birth), transgender women and transgender men who are seeking a PrEP study clinic and age 18-30 years. Study participants must report having anal sex in the last six months with a person assigned male gender at birth.
Health status
Study type
Interventional (clinical trial)
Enrollment
1200
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Methods include qualitative (focus group discussion and in-depth interviews) and quantitative (service statistics, laboratory tests, surveys) approaches. The incidence of HIV in the CAB LA study cohort will be evaluated against a similar cohort receiving oral PrEP. Interrupted time series analysis will be utilised to assess the effectiveness of the mHealth intervention.
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
SEARCH SAPPHIRE DCP Extension
Identifier
NCT05549726
Link
https://clinicaltrials.gov/study/NCT05549726
Phase
Marketed
Status
Active, not recruiting
Sponsor
University of California, San Francisco
More details
Not provided
Purpose
Determine whether adding the option of CAB-LA as HIV PrEP increases prevention coverage compared to the standard-of-care in three ongoing randomized trials of dynamic choice in rural Uganda and Kenya.
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-01-02
Anticipated Date of Last Follow-up
2024-02-23
Estimated Primary Completion Date
Not provided
Estimated Completion Date
2025-01-01
Actual Primary Completion Date
2023-12-18
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adolescents
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Participants must be enrolled in a SEARCH Sapphire Dynamic prevention study (NCT04810650).
Health status
Study type
Interventional (clinical trial)
Enrollment
984
Allocation
Non-randomized
Intervention model
Parallel Assignment
Intervention model description
CAB-LA will be offered to participants that are initially assigned to the Dynamic Choice Prevention delivery model.
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
NCT02478463
Identifier
NCT02478463
Link
https://clinicaltrials.gov/study/NCT02478463
Phase
Phase I
Status
Completed
Sponsor
ViiV Healthcare
More details
Not provided
Purpose
Determine the PK concentrations of CAB following LA administration in relevant tissues and fluids of healthy men and women following a single 600 mg IM dose.
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2017-02-27
Anticipated Date of Last Follow-up
2020-06-02
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2019-07-25
Actual Completion Date
2019-07-25
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: - Between 18 and 55 years of age inclusive, at the time of signing the informed consent. - Healthy as determined by the investigator or medically qualified designee based on a medical evaluation including medical history, physical examination, laboratory tests and cardiac monitoring. - Body weight >= 40 kilogram (kg) and body mass index (BMI) within the range 18.5 to 35 kg /meter square (inclusive). - Male or female. - Capable of giving signed informed consent, which includes compliance with the requirements and restrictions listed in the consent form and in this protocol. - All subjects participating in the study must be counselled on safe sexual practices including the use of effective barrier methods to minimize risk of HIV transmission.
Health status
Study type
Interventional (clinical trial)
Enrollment
19
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
NCT03422172
Identifier
NCT03422172
Link
https://clinicaltrials.gov/study/NCT03422172
Phase
Phase I
Status
Completed
Sponsor
ViiV Healthcare
More details
Not provided
Purpose
Evaluate the PK, safety, tolerability, and acceptability of CAB LA in adult HIV uninfected Chinese male subjects at low risk for HIV acquisition.
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2018-04-10
Anticipated Date of Last Follow-up
2021-05-12
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2020-04-20
Actual Completion Date
2020-04-20
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Cisgender male
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: - Subjects must be 18 to 65 years of age inclusive, at the time of signing the informed consent. - Subjects are male at birth. - Subjects who have non-reactive point of care (POC) HIV test and undetectable HIV-1 ribose nucleic acid (RNA) at screening. - At risk of acquiring HIV, defined as having at least one casual male or female sex partner in the past 24 months. - Healthy as determined by a responsible and experienced physician, based on a medical evaluation including medical history, physical examination, laboratory tests and cardiac monitoring at the time of screening. - Capable of giving written informed consent. - Agree to appropriate use of contraceptive measures during heterosexual intercourse. - Willing to undergo all required study procedures.
Health status
Study type
Interventional (clinical trial)
Enrollment
48
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Eligible subjects will receive oral doses of CAB for 4 weeks followed by IM dosing with CAB LA at Week 5, Week 9, Week 17, Week 25 and Week 33.
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
| Type of key results | Title | Website link |
|---|---|---|
| Article | Safety, Tolerability, Pharmacokinetics, and Acceptability of Oral and Long-Acting Cabotegravir in HIV-Negative Chinese Men | https://doi.org/10.1128/aac.02057-21 |
PALISADE
Identifier
NCT06134362
Link
https://clinicaltrials.gov/study/NCT06134362
Phase
Phase III
Status
Not yet recruiting
Sponsor
ViiV Healthcare
More details
Not provided
Purpose
Long-term follow-up and evaluation of CAB LA for participants in the HPTN 083 and HPTN 084 CAB PrEP studies who are at risk of HIV acquisition.
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2024-04-01
Actual Start Date
Not provided
Anticipated Date of Last Follow-up
2024-01-29
Estimated Primary Completion Date
2027-06-24
Estimated Completion Date
2027-06-24
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Children
- Adolescents
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Yes
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Participants must be currently enrolled and ongoing in one of the following studies: (1) HPTN 083 (2) HPTN 084 (3) HPTN 083 and HPTN 084 adolescent and pregnancy sub-studies Participants who have permanently withdrawn from prior CAB PrEP studies cannot enroll into this study.
Health status
Study type
Interventional (clinical trial)
Enrollment
3500
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
TSHIRELETSO
Identifier
NCT05986084
Link
https://clinicaltrials.gov/study/NCT05986084
Phase
Marketed
Status
Recruiting
Sponsor
Beth Israel Deaconess Medical Center
More details
Not provided
Purpose
aluate whether using long-acting cabotegravir (CAB-LA) for HIV prevention (PrEP) is acceptable, feasible and safe in postpartum people who are breastfeeding.
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-11-30
Anticipated Date of Last Follow-up
2024-07-12
Estimated Primary Completion Date
2027-08-01
Estimated Completion Date
2027-08-31
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Cisgender female
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Yes
Accepts healthy individuals
No
Comments about the studied populations
Participants are postpartum (< 14 days after delivery) mothers who are aged 18-30 with less than a total of three prior pregnancies. Participants should plan to stay and receive postpartum and paediatric care in the Gaborone or Molepolole region of Botswana for at least 24 months.
Health status
Study type
Interventional (clinical trial)
Enrollment
500
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
AXIS
Identifier
NCT06138600
Link
https://clinicaltrials.gov/study/NCT06138600
Phase
Phase III
Status
Completed
Sponsor
University of Witwatersrand, South Africa
More details
This is a mixed methods study employing a convergence model triangulation design. Participants in the study will be sexually active young adults starting Pre-exposure Prophylaxis at private pharmacies, who will be offered either Cabotegravir Long-Acting Injectable, oral Pre-exposure Prophylaxis (TDF/FTC\[3TC\]), or Pre-exposure Prophylaxis deferment at each of their regular visits, with the option to switch between options for up to 15 months, with a final exit interview following the transition to standard-of-care. The number of study visits will vary, depending on participant Pre-exposure Prophylaxis choices. Those choosing oral Pre-exposure Prophylaxis will be seen 3 monthly from V2 onwards, but those choosing Cabotegravir Long-Acting Injectable will be seen 2 monthly from V2. A maximum
Purpose
Acceptability and Feasibility of Injectable Cabotegravir Pre-exposure Prophylaxis (PrEP) Versus Oral PrEP in Routine Care up to 15 Months in Private Pharmacies in South Africa
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-11-01
Anticipated Date of Last Follow-up
2024-06-19
Estimated Primary Completion Date
2025-10-31
Estimated Completion Date
2026-01-31
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion criteria: - Each participant must meet all of the following criteria to be enrolled in this study: 1. Adult male or female (≥18 and ≤ 35 years old) 2. Is self-reported sexually active 3. HIV negative at the time of study enrolment (as determined by a rapid blood test for HIV 1) 4. Body weight ≥ 35 kilograms. 5. Creatinine clearance ≥ 60 mL/min. 6. Willingness to sign informed consent.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
200
Allocation
Non-randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
PEACH
Identifier
NCT05072093
Link
https://clinicaltrials.gov/study/NCT05072093
Phase
Marketed
Status
Active, not recruiting
Sponsor
Emory University
More details
The study is a prospective cohort of young MSM who are followed for 2 years either in-person at the PRISM Health Research Clinic and/or virtually with telehealth study visits. Follow-up visits occur as frequently as every 3 months, or as appropriate to clinical needs of HIV PrEP or STI PEP. The investigators will enroll men who may decide to start or stop PrEP, change from daily oral PrEP to on-demand oral PrEP or from on-demand oral PrEP to daily PrEP, to start or stop STI PEP at any point in the study period, or injectable PrEP as an alternative to daily oral PrEP or on-demand oral PrEP. All men will be provided with the study's mobile smart phone app to support early identification of risks for PrEP discontinuation, to provide information about STI PEP and document usage patterns of on-
Purpose
Parrying the Pitfalls of PrEP: Project PEACH
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-11-20
Anticipated Date of Last Follow-up
2024-06-11
Estimated Primary Completion Date
2025-02-01
Estimated Completion Date
2025-02-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
Genders
- Male
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: * Male at birth * Self-identify as Cisgender Male * Ages 18-45 years * ≥1 male anal sex partner in the 12 months before the baseline interview * Live in the Atlanta MSA * Owns cell phone with data service * Willing to download a health-related app to their cell phone as part of the research study * Able to provide ≥ 2 means of contact * Not currently enrolled in another HIV prevention clinical trial * Confirmed HIV-negative at baseline visit Exclusion Criteria: * Female at birth * Do not self-identify as Cisgender Male * Individuals \< 18 years of age or \> 45 years of age * HIV positive status * No male anal sex partner in the 12 months before the baseline interview * Does not own mobile phone with data service * Not willing to download a health-related app to their
Health status
Study type
Interventional (clinical trial)
Enrollment
240
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
MOBILEMEN
Identifier
NCT06133686
Link
https://clinicaltrials.gov/study/NCT06133686
Phase
Phase III
Status
Not yet recruiting
Sponsor
MRC/UVRI and LSHTM Uganda Research Unit
More details
Title: Implementing oral (event-driven and daily) and long-acting Pre-exposure prophylaxis (PrEP) in mobile men in Sub-Saharan Africa Design: A mixed method, multi-setting, multi-country, phase 3b, open-label, hybrid type 2 implementation and effectiveness randomized controlled trial (RCT). The trial will be carried out in 400 HIV negative men aged 18+ years in South Africa and Uganda. Men will be randomized 1:1 to either Group A: oral Tenofovir disoproxil fumarate/emtricitabine (TDF-FTC) PrEP (event-driven or daily) or Group B: Long-acting injectable cabotegravir (CAB-LA) over 9-months. After 9-months participants from both groups will be offered choice of PrEP (oral TDF-FTC or CAB-LA) for a further 9-months, with the ability to change choice as required. Various strategies to support Pr
Purpose
Implementing Oral (Event-driven and Daily) and Long-acting Pre-Exposure Prophylaxis in Mobile Men in Sub-Saharan Africa
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2024-04-01
Actual Start Date
Not provided
Anticipated Date of Last Follow-up
2023-11-13
Estimated Primary Completion Date
2025-12-31
Estimated Completion Date
2027-04-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
Genders
- Male
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: 1. Able and willing to provide informed consent 2. Aged 18 years and above on the day of screening 3. Willing to have a HIV test and receive the test results 4. Male at birth 5. In the past 6-months has travelled for work or to find work and spent at least one night away from home for work related purposes. 6. Available for follow up for the duration of the study Exclusion Criteria: 1. Known HIV infection 2. Confirmed HIV-positive test result, indeterminate HIV test result, and/or signs and symptoms of an acute HIV infection 3. Body weight less than 35Kg at baseline 4. Allergy to any of the study products 5. Medical, social or other condition that, in the opinion of the site investigator, would interfere with the conduct of the study or safety of the participant (e.g
Health status
Study type
Interventional (clinical trial)
Enrollment
400
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
PathToScale
Identifier
NCT06319105
Link
https://clinicaltrials.gov/study/NCT06319105
Phase
Marketed
Status
Recruiting
Sponsor
Georgetown University
More details
The purpose of this study is to evaluate the implementation and clinical outcomes of expanded pre-exposure prophylaxis delivery modalities and service delivery points offering long-acting injectable cabotegravir and oral pre-exposure prophylaxis to high-priority groups through diverse delivery channels.
Purpose
PathToScale: An Implementation Evaluation
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2024-03-26
Anticipated Date of Last Follow-up
2024-03-28
Estimated Primary Completion Date
2026-04-01
Estimated Completion Date
2026-04-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
Unspecified
Genders
Unspecified
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Unspecified
Comments about the studied populations
Not provided
Health status
Not provided
Study type
Observational studies (incl. patient registries)
Enrollment
9900
Allocation
Non-randomized
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
IDCaPP
Identifier
NCT05867212
Link
https://clinicaltrials.gov/study/NCT05867212
Phase
Marketed
Status
Active, not recruiting
Sponsor
Kelley-Ross & Associates, Inc.
More details
The goal of this demonstration project or observational study is to evaluate the feasibility and acceptability of a pharmacist-managed cabotegravir long acting injectable for PrEP program in a community pharmacy setting. The main question it aims to answer are: * Is the program feasible and acceptable at the end of 1 year of operations? * What are the facilitators and barriers of the program? Participants who want to start the FDA approved cabotegravir long acting injectable medication for PrEP will have the option participating in surveys and a review of their electronic health records. Medication will be administered based on FDA approved labeling guidelines and their PrEP care will be part of standard of care per CDC. Pharmacists who want to provide the service to their patients will
Purpose
Implementation and Delivery of Cabotegravir Long Acting Injection for PrEP in a Community Pharmacy Setting.
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2023-06-01
Actual Start Date
Not provided
Anticipated Date of Last Follow-up
2023-05-16
Estimated Primary Completion Date
2025-11-30
Estimated Completion Date
2025-11-30
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
Yes
Accepts lactating individuals
Yes
Accepts healthy individuals
Yes
Comments about the studied populations
Not provided
Health status
Study type
Observational studies (incl. patient registries)
Enrollment
50
Allocation
Non-randomized
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
HIPCY
Identifier
NCT06474364
Link
https://clinicaltrials.gov/study/NCT06474364
Phase
Marketed
Status
Active, not recruiting
Sponsor
MU-JHU CARE
More details
Several studies show that Adolescents and Young Adults (AYA) have poor outcomes along the entire Human Immunodeficiency Virus (HIV) prevention and care cascades compared to adults. The investigators propose to evaluate novel evidence-based HIV prevention and care interventions (including Cabotegravir LongActing (CABLA) to determine implementation outcomes among AYA who are at particularly high risk for HIV acquisition and poor viral suppression in five geographically distinct research performance sites in Uganda. The results will provide important evidence to inform Uganda and other regional countries' policy on integrated HIV prevention, care and treatment for AYA at high risk for HIV and Sexually Transmitted Infections (STIs) in order to reach the UNAIDS 95-95-95 targets and HIV epidemic
Purpose
HIV Prevention and Care Interventions for Youth in Uganda
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2024-07-15
Actual Start Date
Not provided
Anticipated Date of Last Follow-up
2024-06-19
Estimated Primary Completion Date
2028-08-31
Estimated Completion Date
2028-08-31
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adolescents
- Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: Adolescents and young adults with increased likelihood of HIV acquisition * AYA 15 to 24 years of age * Classified as high risk using our screening tool. * Documented HIV un-infected as per the national HIV testing algorithm. * Willing to use PrEP * Willing to provide written informed consent. * No plans to relocate permanently in the next 6 months * No suspicion of acute HIV infection: * Hepatitis B virus surface antigen (HBsAg)-negative and accepts HB vaccination * Having no medical or social condition that, in the opinion of the study investigator, would interfere with the conduct of the study or interpretation of study results. * HIV-uninfected, based on HIV test results obtained at screening and enrolment visit and just prior to randomization. All HIV test res
Health status
Study type
Interventional (clinical trial)
Enrollment
600
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
CATALYST
Identifier
NCT05937698
Link
https://clinicaltrials.gov/study/NCT05937698
Phase
Marketed
Status
Recruiting
Sponsor
FHI 360
More details
The CATALYST study is an implementation study that will characterize and assess the implementation of an enhanced service delivery package providing informed choice of pre-exposure prophylaxis (PrEP) products among women at PEPFAR sites in Kenya, Lesotho, South Africa, Uganda, and Zimbabwe.
Purpose
The CATALYST Study
Interventions
Not provided
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-05-30
Anticipated Date of Last Follow-up
2024-07-29
Estimated Primary Completion Date
2025-06-30
Estimated Completion Date
2025-06-30
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
Unspecified
Genders
Unspecified
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Unspecified
Comments about the studied populations
Not provided
Health status
Not provided
Study type
Observational studies (incl. patient registries)
Enrollment
11256
Allocation
Non-randomized
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
LAPIS
Identifier
NCT06250504
Link
https://clinicaltrials.gov/study/NCT06250504
Phase
Phase III
Status
Recruiting
Sponsor
Africa Health Research Institute
More details
The goal of this hybrid (1a) Cluster Randomised Controlled Trial phase 3B trial is to evaluate the effectiveness and implementation of offering a choice of HIV Pre-Exposure Products (PrEP) through community-based sexual and reproductive health services, on PrEP uptake and retention, and population prevalence of sexually transmissible HIV amongst adolescents and young adults living in rural South Africa. Researchers will compare adding the choice of long-acting PrEP, i.e. two monthly injectable cabotegravir (CAB LA) or dapiravine vaginal ring and HIV post exposure prophylaxis packs to daily oral PrEP integrated with community-based SRH in the 20 intervention clusters with standard of care (SoC), daily oral PrEP integrated with community-based SRH in the 20 control clusters, on uptake and r
Purpose
Long-Acting HIV Pre-Exposure Prophylaxis Integrated With Sexual and Reproductive Health - cRCT
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2024-02-27
Anticipated Date of Last Follow-up
2024-04-16
Estimated Primary Completion Date
2025-07-01
Estimated Completion Date
2026-03-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Children
- Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: All young men and women aged 15-30 who are residing in the 40 administrative clusters in the study district and attend any integrated SRH/HIV service Documented HIV negative test Suitable for PrEP and/or already on PrEP Weight \> 35 kg Understand the required dosing schedule and HIV testing. Aware that details can be shared with a peer navigator to support their follow-up If pregnant or breast feeding and/or planning to become pregnant participant can be offered CAB LA, if risk of acquiring HIV out weighs unknown risk of CAB LA, but must understand that safety in pregnancy or breast feeding for CAB LA has not been established and oral daily PrEP is a safe alternative. Exclusion Criteria: History or presence of allergy to the study drugs or their components Inv
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
2000
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Double-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
TIARAS-CAB-WWID
Identifier
NCT05799339
Link
https://clinicaltrials.gov/study/NCT05799339
Phase
Marketed
Status
Recruiting
Sponsor
Alexis Roth
More details
The goal of this study is to elicit information crucial for designing strategies to support engagement in cabotegravir, a long-acting injectable form of pre-exposure prophylaxis (PrEP) to reduce HIV risk among women who inject drugs (WWID), a population with high unmet need that has been understudied in all phases of PrEP research. The main questions this study aims to answer are: 1. How do WWID perceive long-acting injectable cabotegravir (CAB-LA) as a HIV prevention tool? 2. If and how their decisions to initiate CAB-LA as PrEP are informed by their experiences with other long-acting medications, experience with daily oral medications, and their personal circumstance (e.g., like housing or addition severity)? 3. Do PrEP outcomes (e.g., adherence) and engagement in care over time differ
Purpose
Optimizing CAB-LA as PrEP for Women Who Inject Drugs
Interventions
Not provided
Countries
Not provided
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-01-13
Anticipated Date of Last Follow-up
2024-03-01
Estimated Primary Completion Date
2024-12-01
Estimated Completion Date
2024-12-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
Genders
- Female
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Women who inject drugs
Health status
Study type
Observational studies (incl. patient registries)
Enrollment
144
Allocation
Non-randomized
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
AXIS
Identifier
NCT06138600
Link
https://clinicaltrials.gov/study/NCT06138600
Phase
Phase III
Status
Active, not recruiting
Sponsor
University of Witwatersrand, South Africa
More details
This is a mixed methods study employing a convergence model triangulation design. Participants in the study will be sexually active young adults starting Pre-exposure Prophylaxis at private pharmacies, who will be offered either Cabotegravir Long-Acting Injectable, oral Pre-exposure Prophylaxis (TDF/FTC\[3TC\]), or Pre-exposure Prophylaxis deferment at each of their regular visits, with the option to switch between options for up to 15 months, with a final exit interview following the transition to standard-of-care. The number of study visits will vary, depending on participant Pre-exposure Prophylaxis choices. Those choosing oral Pre-exposure Prophylaxis will be seen 3 monthly from V2 onwards, but those choosing Cabotegravir Long-Acting Injectable will be seen 2 monthly from V2. A maximum
Purpose
Acceptability and Feasibility of Injectable Cabotegravir Pre-exposure Prophylaxis (PrEP) Versus Oral PrEP in Routine Care up to 15 Months in Private Pharmacies in South Africa
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-11-01
Anticipated Date of Last Follow-up
2024-06-19
Estimated Primary Completion Date
2025-10-31
Estimated Completion Date
2026-01-31
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: - Each participant must meet all of the following criteria to be enrolled in this study: 1. Adult male or female (≥18 and ≤ 35 years old) 2. Is self-reported sexually active 3. HIV negative at the time of study enrolment (as determined by a rapid blood test for HIV 1) 4. Body weight ≥ 35 kilograms. 5. Creatinine clearance ≥ 60 mL/min. 6. Willingness to sign informed consent. Exclusion Criteria: - Participants meeting the following criteria will be excluded from participating in the study: 1. Symptoms of HIV seroconversion (see Table 1). 2. Pregnant (participant must have a negative beta human chorionic gonadotrophin (b-hCG) urine test at screening) or lactating women, or women intending to become pregnant or breastfeed during the study. 3. Is in good health, with
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
200
Allocation
Not provided
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
Excipients
Proprietary excipients used
No proprietary excipient used
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
No novel excipient or existing excipient used
Residual solvents used
No residual solvent used
Patent info
Compound patent families
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Cabotegravir crystalline forms
Expiry date: 2038-01-25 A crystalline form and pharmaceutical compositions comprising it |
WO2018149608 | Polymorphs | Sandoz Ag | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Mexico | Australia, United Kingdom, France, Germany, United States of America |
| Filed | China | Canada |
| Not in force | World Intellectual Property Organization (WIPO), Morocco, Albania, Serbia, Bosnia and Herzegovina, Montenegro, Türkiye, Moldova, Republic of, North Macedonia, Tunisia | World Intellectual Property Organization (WIPO), Liechtenstein, Italy, Norway, Malta, Denmark, Belgium, Greece, Netherlands, Hungary, Croatia, Switzerland, Spain, San Marino, Slovenia, Austria, Romania, Iceland, Cyprus, Finland, Bulgaria, Slovakia, Poland, Latvia, Ireland, Estonia, Luxembourg, Portugal, Czechia, Lithuania, Monaco, Sweden, Russian Federation, United States of America |
MPP Licence(s)
MPP Licence on Cabotegravir (tablet form and/or long-acting injectable form) for HIV pre-exposure prophylaxis (PrEP)
https://medicinespatentpool.org/licence-post/cabotegravir-long-acting-la-for-hiv-pre-exposure-prophylaxis-prepPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Cabotegravir long-acting parenteral compositions
Expiry date: 2031-09-15 The present invention relates to pharmaceutical compositions of cabotegravir useful in the treatment or prevention of Human Immunodeficiency Virus (HIV) infections. |
WO2012037320 | Composition | Glaxosmithkline Llc, Mundhra, Deepak B, Pan, Rennan, Viiv Healthcare Company | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Brazil, China, Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Moldova, Republic of, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro, Serbia, Mexico, Ukraine, South Africa, India | Australia, Canada, Chile, Russian Federation, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania, Slovenia, Israel, Japan, Korea, Republic of, Taiwan, Province of China, United States of America |
| Filed | United States of America | |
| Not in force | World Intellectual Property Organization (WIPO) | World Intellectual Property Organization (WIPO), United States of America |
MPP Licence(s)
MPP Licence on Cabotegravir (tablet form and/or long-acting injectable form) for HIV pre-exposure prophylaxis (PrEP)
https://medicinespatentpool.org/licence-post/cabotegravir-long-acting-la-for-hiv-pre-exposure-prophylaxis-prepPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Cabotegravir processes and intermediates
Expiry date: 2031-03-22 Relates to the preparation of carbamoylpyridone derivatives and intermediates which are useful as HIV integrase inhibitors. |
WO2011119566 | Intermediate(s), Process | Glaxosmithkline Llc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | China, Albania, Serbia, Bosnia and Herzegovina, Montenegro, Türkiye, North Macedonia, India | Liechtenstein, Italy, Norway, Malta, Denmark, Belgium, United Kingdom, Greece, Netherlands, Hungary, Croatia, Switzerland, Spain, San Marino, Slovenia, Austria, Romania, Iceland, Cyprus, Finland, France, Bulgaria, Slovakia, Poland, Latvia, Ireland, Estonia, Germany, Luxembourg, Portugal, Czechia, Lithuania, Monaco, Sweden, Japan, Korea, Republic of, United States of America |
| Filed | San Marino, Singapore, Taiwan, Province of China | |
| Not in force | World Intellectual Property Organization (WIPO) | World Intellectual Property Organization (WIPO) |
MPP Licence(s)
MPP Licence on Cabotegravir (tablet form and/or long-acting injectable form) for HIV pre-exposure prophylaxis (PrEP)
https://medicinespatentpool.org/licence-post/cabotegravir-long-acting-la-for-hiv-pre-exposure-prophylaxis-prepPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Dolutegravir and Cabotegravir compounds
Expiry date: 2026-04-28 The present invention is to provide a novel compound (I), having the anti-virus activity, particularly the HIV integrase inhibitory activity, and a drug containing the same, particularly an anti-HIV drug, as well as a process and an intermediate thereof. Compound (I) wherein Z<1> is NR<4>; R<1> is hydrogen or lower alkyl; X is a single bond, a hetero atom group selected from O, S, SO, SO2 and NH, or lower alkylene or lower alkenylene in which the hetero atom group may intervene; R<2> is optionally substituted aryl; R<3> is hydrogen, a halogen, hydroxy, optionally substituted lower alkyl etc; and R<4> and Z<2> part taken together forms a ring, to form a polycyclic compound, including e.g., a tricyclic or tetracyclic compound. |
WO2006116764 | Compound | Glaxosmithkline Llc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Brazil, China, Morocco, Mexico, Philippines, Ukraine, Viet Nam, South Africa, Türkiye, Armenia, Azerbaijan, Belarus, Kyrgyzstan, Kazakhstan, Moldova, Republic of, Tajikistan, Turkmenistan, Nigeria, Colombia, Indonesia, Malaysia, Algeria | United States of America, Australia, Canada, Cyprus, Hong Kong, Israel, Japan, Korea, Republic of, Luxembourg, Norway, New Zealand, Taiwan, Province of China, Austria, Belgium, Bulgaria, Switzerland, Czechia, Germany, Denmark, Estonia, Spain, Finland, France, United Kingdom, Greece, Hungary, Ireland, Iceland, Italy, Liechtenstein, Lithuania, Latvia, Monaco, Netherlands, Poland, Portugal, Romania, Sweden, Slovenia, Slovakia, Russian Federation, Trinidad and Tobago, Singapore |
| Filed | Egypt | United States of America, Cyprus, Luxembourg, Norway, Finland, France, Hungary, Lithuania, Netherlands, Slovenia |
| Not in force | Türkiye, India, World Intellectual Property Organization (WIPO) | United States of America, Cyprus, Hong Kong, Israel, Japan, Luxembourg, Austria, Belgium, Bulgaria, Switzerland, Czechia, Germany, Denmark, Estonia, Spain, Finland, France, United Kingdom, Greece, Hungary, Ireland, Iceland, Italy, Liechtenstein, Lithuania, Latvia, Monaco, Netherlands, Poland, Portugal, Romania, Sweden, Slovenia, Slovakia, World Intellectual Property Organization (WIPO) |
MPP Licence(s)
MPP Licence on Cabotegravir (tablet form and/or long-acting injectable form) for HIV pre-exposure prophylaxis (PrEP)
https://medicinespatentpool.org/licence-post/cabotegravir-long-acting-la-for-hiv-pre-exposure-prophylaxis-prepSupporting material
Publications
Bowers GD, Culp A, Reese MJ, Tabolt G, Moss L, Piscitelli S, Huynh P, Wagner D, Ford SL, Gould EP, Pan R, Lou Y, Margolis DA, Spreen WR: Disposition and metabolism of cabotegravir: a comparison of biotransformation and excretion between different species and routes of administration in humans. Xenobiotica. 2016;46(2):147-62. doi: https://doi.org/10.3109/00498254.2015.1060372 Epub 2015 Jul 1
1. Cabotegravir [(3S,11aR)-N-[(2,4-difluorophenyl)methyl]-6-hydroxy-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydro[1,3]oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide] is an HIV-1 integrase inhibitor under development as a tablet for both oral lead-in therapy and long-acting (LA) injectable for intramuscular dosing.
2. Metabolism, pharmacokinetics and excretion were investigated in healthy human subjects who received either a single oral dose (28.2 mg) of [14C]cabotegravir in a mass balance study, or LA formulations of unlabeled cabotegravir (200–800 mg), intramuscularly or subcutaneously, in a separate study. Metabolism, distribution and excretion of [14C]cabotegravir were also investigated in mice, rats and monkeys.
3. Recovery of radioactivity in humans represented a mean total of 85.3% of the dose, including 26.8% in the urine. The mean apparent terminal phase half-life was similar for both cabotegravir and radioactivity, 39 h compared to 41 h.
4. Following oral, intramuscular and subcutaneous administration, cabotegravir was the major component in plasma and the glucuronic acid conjugate (M1) represented the predominant component in urine. Cabotegravir was present in bile along with its major metabolite (M1).
5. The primary metabolite of [14C]cabotegravir in mouse, rat and monkey was the same as that in human. In vitro phenotyping experiments demonstrated that cabotegravir was metabolized by UDP-glucuronosyltransferase (UGT) 1A1 and UGT1A9.
Spreen W, Min S, Ford SL, Chen S, Lou Y, Bomar M, St Clair M, Piscitelli S, Fujiwara T: Pharmacokinetics, safety, and monotherapy antiviral activity of GSK1265744, an HIV integrase strand transfer inhibitor. HIV Clin Trials. 2013 Sep-Oct;14(5):192-203. doi: https://doi.org/10.1310/hct1405-192
Background: GSK1265744 is an HIV integrase strand transfer inhibitor selected for clinical development.
Objective: This first-time-in-human and phase IIa investigation assessed GSK1265744 antiviral activity, pharmacokinetics, safety, and tolerability in healthy and HIV-1-infected subjects.
Methods: This double-blind, placebo-controlled study consisted of a dose escalation of single (part A) and multiple (part B) oral doses in 48 healthy subjects and an oral dose (part C) in 11 HIV-1-infected subjects. In part A, 2 cohorts of 9 subjects received either 5 and 25 mg or 10 and 50 mg. In part B, 3 cohorts of 10 subjects received 5, 10, or 25 mg once daily for 14 days. In part C and the phase IIa study, subjects received 5 or 30 mg once daily for 10 days.
Results: Dose-proportional increases in drug exposure were observed in healthy and HIV-1-infected subjects. In healthy subjects, pharmacokinetic variability was low following single or repeat dosing (coefficient of variation, 13%-34% and 15%-23%, respectively). Mean plasma half-life was 31.5 hours. GSK1265744 monotherapy significantly reduced plasma HIV-1 RNA from baseline to day 11 in HIV-1-infected subjects receiving 5 or 30 mg versus placebo (P < .001); mean decrease was 2.2 to 2.3 log10 copies/mL, respectively. Study drug was generally well tolerated with no clinically relevant trends in laboratory values, vital signs, or electrocardiograms.
Conclusions: GSK1265744 was well tolerated in healthy and HIV-1-infected subjects. Results demonstrate once-daily doses of 5 or 30 mg exceeded minimum target therapeutic concentrations and produced a significant reduction in plasma HIV-1 RNA viral load.
Trezza C, Ford SL, Spreen W, Pan R, Piscitelli S. Formulation and pharmacology of long-acting cabotegravir. Curr Opin HIV AIDS. 2015 Jul;10(4):239-45. doi: https://doi.org/10.1097%2FCOH.0000000000000168. PMID: 26049948; PMCID: PMC5638427.
Purpose of review
Long-acting cabotegravir may provide a novel therapeutic option for both the treatment and prevention of HIV-1 infection that does not necessitate adherence to a daily regimen. The present review will highlight the unique formulation properties and pharmacologic attributes of long-acting cabotegravir nanosuspension.
Recent findings
Cabotegravir is a potent integrase strand transfer inhibitor that has been formulated as an oral tablet for daily administration and as a long-acting injectable nanosuspension. Long-acting cabotegravir is readily absorbed following intramuscular and subcutaneous administration and has an elimination half-life of approximately 40 days, allowing for administration on a monthly or less frequent schedule. Repeat-dose pharmacokinetic studies and population pharmacokinetic modeling indicate monthly and bi-monthly dosing achieves clinically relevant plasma concentrations considered effective for HIV maintenance therapy and that quarterly injections are appropriate for investigation as preexposure prophylaxis. Cabotegravir is primarily metabolized by uridine diphosphate glucuronosyltransferase 1A1 and is unlikely to be impacted by the cytochrome P450 metabolic pathway. In vitro and in vivo data suggest cabotegravir has a low propensity to cause, or be subject to, significant drug interactions.
Summary
The pharmacologic profile of long-acting cabotegravir supports its continued development for both treatment and prevention of HIV-1 infection.
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Not provided