
|
Developed by 

|
Supported by 

|
ELQ-331
Developer(s)

|
Oregon Health Sciences University Originator
https://www.ohsu.edu/
United States Oregon Health & Science University (OHSU) is Oregon's premier public research institution dedicated to health sciences. Established in 1887, OHSU boasts a renowned research environment tackling critical health challenges across neuroscience, cancer biology & infectious diseases. Nationally recognized for its research output, its collaborative environment fosters innovation in the healthcare space. |

|
Medicines for Malaria Ventures Originator
https://www.mmv.org/
Switzerland MMV is a Swiss-based not-for-profit organization working through a product development partnership model to deliver a portfolio of accessible medicines with the power to treat, prevent and eliminate malaria. We close critical gaps in research, development and access – working “end-to-end” to expand the use of existing antimalarials and innovate new compounds to protect public health. |
Drug structure

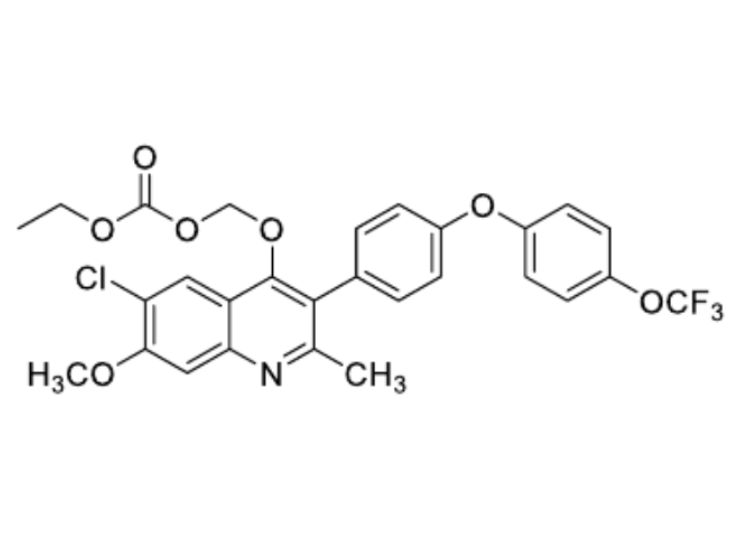
ELQ-331 Chemical Structure
https://doi.org/10.1186/s12936-019-2921-9
Drug information
Associated long-acting platforms
Polymer-based particles, Aqueous drug particle suspension
Administration route
Intramuscular, Oral
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
User acceptance
Not provided
Dosage
Available dose and strength
Not provided
Frequency of administration
Not provided
Maximum dose
Not provided
Recommended dosing regimen
Not provided
Additional comments
Not provided
Dosage link(s)
Not provided
Drug information
Drug's link(s)
Not provided
Generic name
Brand name
Compound type
Summary
Approval status
Regulatory authorities
Delivery device(s)
No delivery device
Scale-up and manufacturing prospects
Scale-up prospects
ELQ-331 can be formulated utilising spray-dried dispersion (SDD) or self-emulsifying drug delivery system (SEDDS) approaches to improve solubility and bioavailability. Spray-drying technology is amenable to production scale-up and is suitable for continuous processing, which could potentially facilitate the industrial manufacture of ELQ-331. The SEDDS formulation method displayed a narrow particle distribution, small emulsion droplet sizes & good self-emulsifying properties. Testing of SEDDS formulations in rat models showed a 1.4-fold increase in ELQ-300 levels compared with the SDD approach.
Tentative equipment list for manufacturing
For SDD Formulations: (1) Industrial-scale spray-dryer, (2) Scintillation vials, (3) Laboratory mixer, (4) Laboratory desiccator and (5) Soluplus® (Polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft co-polymer). For SEDDS Formulations: (1) Titration apparatus, (2) Vortex mixer and (3) Aeroperl® 300 Pharma (Colloidal silicon dioxide).
Manufacturing
Spray-dried formulations of ELQ-331 were created through dissolution of the drug and polymer (Soluplus®) in organic solvent. Following this, insoluble granulated high purity colloidal silicon dioxide (Aeroperl® 300 Pharma) was dispersed and spray dried into a powder. The SDD powder is shelf stable and showed no signs of degradation after ambient storage for twelve weeks under closed and open cap conditions. SEDDS formulations were achieved by assessing ELQ-331 solubility in surfactants, co-surfactants and oils. From here, a suitable combination of premix was identified for SEDDS generation.
Specific analytical instrument required for characterization of formulation
For SDD Formulation: (1) HPLC to assess drug content. (2) Fourier transform infrared spectroscopy to detect chemical changes. (3) Differential scanning calorimetry and Powder X-ray diffraction (PXRD) to identify presence/absence of crystallinity. (4) Thermogravimetric analysis for thermal stability studies. For SEDDS Formulation: (1) Dynamic light scattering nanoparticle size analyzer (e.g. Horiba LB-550, Sunnyvale, CA) to determine droplet size and distribution. (2) HPLC to characterise the overall formulation.
Clinical trials
Not providedExcipients
Proprietary excipients used
No proprietary excipient used
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
No novel excipient or existing excipient used
Residual solvents used
No residual solvent used
Patent info
Description
ELQ-331 compound as broad Markush structure
Brief description
Disclosed are derivative compounds of ELQ-300 that include an ester at position 4. These compounds have enhanced properties relative to ELQ-300. Also disclosed are pharmaceutical compositions comprising the compounds and methods of treating and preventing malaria infections involving administering the pharmaceutical compositions to the subject.
Representative patent
WO2017015360
Category
Compound
Patent holder
Univ Oregon Health & Science; US Gov Veterans Affairs
Exclusivity
Not provided
Expiration date
July 27, 2036
Status
Only granted in the US (US10584098B2)
Supporting material
Publications
Smilkstein MJ, Pou S, Krollenbrock A, Bleyle LA, Dodean RA, Frueh L, Hinrichs DJ, Li Y, Martinson T, Munar MY, Winter RW, Bruzual I, Whiteside S, Nilsen A, Koop DR, Kelly JX, Kappe SHI, Wilder BK, Riscoe MK. ELQ-331 as a prototype for extremely durable chemoprotection against malaria. Malar J. 2019 Aug 27;18(1):291. doi: 10.1186/s12936-019-2921-9. PMID: 31455339; PMCID: PMC6712883.
Background: The potential benefits of long-acting injectable chemoprotection (LAI-C) against malaria have been recently recognized, prompting a call for suitable candidate drugs to help meet this need. On the basis of its known pharmacodynamic and pharmacokinetic profiles after oral dosing, ELQ-331, a prodrug of the parasite mitochondrial electron transport inhibitor ELQ-300, was selected for study of pharmacokinetics and efficacy as LAI-C in mice.
Methods: Four trials were conducted in which mice were injected with a single intramuscular dose of ELQ-331 or other ELQ-300 prodrugs in sesame oil with 1.2% benzyl alcohol; the ELQ-300 content of the doses ranged from 2.5 to 30 mg/kg. Initial blood stage challenges with Plasmodium yoelii were used to establish the model, but the definitive study measure of efficacy was outcome after sporozoite challenge with a luciferase-expressing P. yoelii, assessed by whole-body live animal imaging. Snapshot determinations of plasma ELQ-300 concentration ([ELQ-300]) were made after all prodrug injections; after the highest dose of ELQ-331 (equivalent to 30 mg/kg ELQ-300), both [ELQ-331] and [ELQ-300] were measured at a series of timepoints from 6 h to 5½ months after injection.
Results: A single intramuscular injection of ELQ-331 outperformed four other ELQ-300 prodrugs and, at a dose equivalent to 30 mg/kg ELQ-300, protected mice against challenge with P. yoelii sporozoites for at least 4½ months. Pharmacokinetic evaluation revealed rapid and essentially complete conversion of ELQ-331 to ELQ-300, a rapidly achieved (< 6 h) and sustained (4-5 months) effective plasma ELQ-300 concentration, maximum ELQ-300 concentrations far below the estimated threshold for toxicity, and a distinctive ELQ-300 concentration versus time profile. Pharmacokinetic modeling indicates a high-capacity, slow-exchange tissue compartment which serves to accumulate and then slowly redistribute ELQ-300 into blood, and this property facilitates an extremely long period during which ELQ-300 concentration is sustained above a minimum fully-protective threshold (60-80 nM).
Conclusions: Extrapolation of these results to humans predicts that ELQ-331 should be capable of meeting and far-exceeding currently published duration-of-effect goals for anti-malarial LAI-C. Furthermore, the distinctive pharmacokinetic profile of ELQ-300 after treatment with ELQ-331 may facilitate durable protection and enable protection for far longer than 3 months. These findings suggest that ELQ-331 warrants consideration as a leading prototype for LAI-C.
Frueh L, Li Y, Mather MW, Li Q, Pou S, Nilsen A, Winter RW, Forquer IP, Pershing AM, Xie LH, Smilkstein MJ, Caridha D, Koop DR, Campbell RF, Sciotti RJ, Kreishman-Deitrick M, Kelly JX, Vesely B, Vaidya AB, Riscoe MK. Alkoxycarbonate Ester Prodrugs of Preclinical Drug Candidate ELQ-300 for Prophylaxis and Treatment of Malaria. ACS Infect Dis. 2017 Oct 13;3(10):728-735. doi: 10.1021/acsinfecdis.7b00062. Epub 2017 Sep 27. PMID: 28927276; PMCID: PMC5947850.
ELQ-300 is a preclinical antimalarial drug candidate that is active against liver, blood, and transmission stages of Plasmodium falciparum. While ELQ-300 is highly effective when administered in a low multidose regimen, poor aqueous solubility and high crystallinity have hindered its clinical development. To overcome its challenging physiochemical properties, a number of bioreversible alkoxycarbonate ester prodrugs of ELQ-300 were synthesized. These bioreversible prodrugs are converted to ELQ-300 by host and parasite esterase action in the liver and bloodstream of the host. One such alkoxycarbonate prodrug, ELQ-331, is curative against Plasmodium yoelii with a single low dose of 3 mg/kg in a murine model of patent malaria infection. ELQ-331 is at least as fully protective as ELQ-300 in a murine malaria prophylaxis model when delivered 24 h before sporozoite inoculation at an oral dose of 1 mg/kg. Here, we show that ELQ-331 is a promising prodrug of ELQ-300 with improved physiochemical and metabolic properties and excellent potential for clinical formulation.
Potharaju S, Mutyam SK, Liu M, Green C, Frueh L, Nilsen A, Pou S, Winter R, Riscoe MK, Shankar G. Improving solubility and oral bioavailability of a novel antimalarial prodrug: comparing spray-dried dispersions with self-emulsifying drug delivery systems. Pharm Dev Technol. 2020 Jun;25(5):625-639. doi: 10.1080/10837450.2020.1725893. Epub 2020 Feb 12. PMID: 32031478; PMCID: PMC8120996.
To improve the solubility and oral bioavailability of a novel antimalarial agent ELQ-331 (a prodrug of ELQ-300), spray-dried dispersions (SDD) and a self-emulsifying drug delivery system (SEDDS) were developed. SDD were prepared with polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer (Soluplus®) polymer carrier and Aeroperl® 300 Pharma and characterized by differential scanning calorimetry, powder X-ray diffraction. For SEDDS, solubility in oils, surfactants, and co-surfactants was determined and ternary phase diagram was constructed to show self-emulsifying area. SEDDS were characterized for spontaneous emulsification and droplet size distribution. The amorphous ELQ-331 SDD improved the solubility to 10× in fast-state simulated intestinal fluid and addition of sodium lauryl sulphate externally to SDDs further improved the solubility to ∼28.5× versus non-formulated drug. SEDDS had good self-emulsifying characteristics with small emulsion droplet sizes and narrow particle distribution. Oral pharmacokinetic studies for SDD and SEDDS formulations were performed in rats. The ELQ-331 rapidly converted to ELQ-300 soon after oral administration in rats. Exposure levels of ELQ-300 were about 1.4-fold higher (based on AUC) in SEDDS than SDD formulations. Poorly soluble drugs like ELQ-331 can be formulated using SDD or SEDDS to improve solubility and oral bioavailability.
Karunakaran D, Mutyam SK, Fu M, et al. Long-acting intramuscular injections of ELQ-331, an antimalarial agent. Eur J Pharm Sci. 2024;198:106795. doi:10.1016/j.ejps.2024.106795
The overarching premise of this investigation is that injectable, long-acting antimalarial medication would encourage adherence to a dosage regimen for populations at risk of contracting the disease. To advance support for this goal, we have developed oil-based formulations of ELQ-331 (a prodrug of ELQ-300) that perform as long-acting, injectable chemoprophylactics with drug loading as high as 160 mg/ml of ELQ-331. In a pharmacokinetic study performed with rats, a single intramuscular injection of 12.14 mg/kg maintained higher plasma levels than the previously established minimum fully protective plasma concentration (33.25 ng/ml) of ELQ-300 for more than 4 weeks. The formulations were well tolerated by the rats and the tested dose produced no adverse reactions. We believe that by extending the length of time between subsequent injections, these injectable oil-based solutions of ELQ-331 can offer a more accessible, low-cost option for long-acting disease prevention and reduced transmission in malaria-endemic regions and may also be of use to travelers.
Additional documents
No documents were uploaded
Useful links
There are no additional links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Not provided