
|
Developed by 

|
Supported by 

|

Glecaprevir and pibrentasvir (G/P)
Developer(s)

|
AbbVie Originator
https://www.abbvie.com/
United States AbbVie Inc. is a global biopharmaceutical company that manufactures and develops innovative medicines as part of a diversified portfolio across several therapeutic categories including immunology, oncology, neuroscience, aesthetics and eyecare. Headquartered in North Chicago, Illinois, AbbVie was founded in 2013 following a successful corporate spin-off from its parent company Abbott Laboratories. |
Drug structure
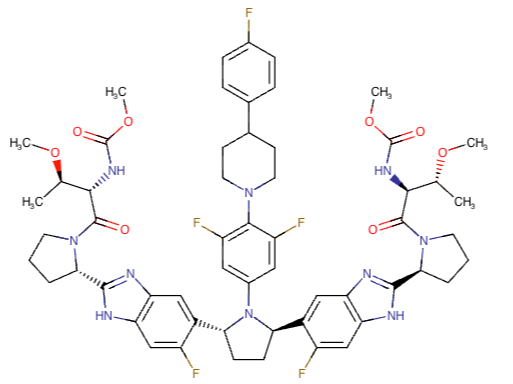
Pibrentasvir Chemical Structure
Sourced from Drugbank
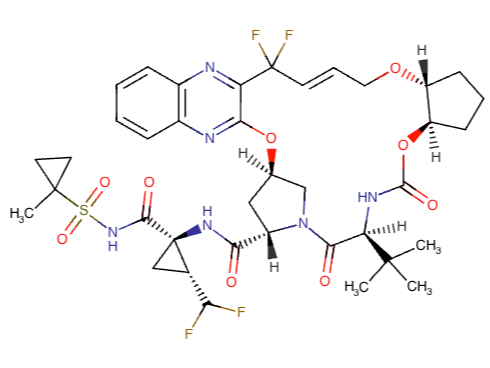
Glecaprevir Chemical Structure
Sourced from Drugbank

Glecaprevir and Pibrentasvir Chemical Structures
Drug information
Associated long-acting platforms
Aqueous drug particle suspension
Administration route
Oral, Intramuscular
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
Frequency of administration
Not provided
User acceptance
Not provided
Dosage
Available dose and strength
Not provided
Maximum dose
Not provided
Recommended dosing regimen
Not provided
Additional comments
Not provided
Dosage link(s)
Not provided
Drug information
Drug's link(s)
Not provided
Generic name
Brand name
Compound type
Drug class/category
Summary
Approval status
Regulatory authorities
Delivery device(s)
Not provided
Scale-up and manufacturing prospects
Scale-up prospects
Long-acting formulations of Glecaprevir and Pibrentasvir (G/P) are still in the early stages of drug development and therefore manufacturing information is limited with few reported examples. One novel approach currently being pioneered by researchers at Tandem Nano Ltd. utilises a proprietary Solid Drug Nanoparticle (SDN) technology platform to achieve high levels of G/P drug loading (>500mg/mL). Interestingly, pre-clinical pharmacokinetic assessments displayed an apparent difference in the release kinetics of both drugs which could be related to differences in aqueous solubility.
Tentative equipment list for manufacturing
Not provided
Manufacturing
Proposed minimally acceptable characteristics for prospective long-acting G/P formulations include: (1) 12-month shelf life as a powder with no cold chain required, (2) suitable drug volume enabling a one monthly intramuscular injection, (3) manageable injection site reaction, and (4) cost equal or less than the oral therapy equivalent.
Specific analytical instrument required for characterization of formulation
Not provided
Clinical trials
Not providedExcipients
Proprietary excipients used
Not provided
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
Not provided
Residual solvents used
Not provided
Patent info
Compound patent families
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Glecaprevir/Pibrentasvir use in HCV (without IFN or RBV) - treatment regimen
Expiry date: 2038-02-09 The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents to a subject with HCV infection, wherein the treatment lasts for 12 weeks and does not include administration of either interferon or ribavirin, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof. |
CA2994496 | Use | Abbvie Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | United States of America | |
| Filed | Canada | |
| Not in force | China, Brazil, Mexico, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro, Serbia, Moldova, Republic of, Morocco, Tunisia | Australia, Japan, United States of America, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania, Slovenia |
MPP Licence(s)
MPP licence on Glecaprevir/Pibrentasvir (G/P)
https://medicinespatentpool.org/licence-post/glecaprevir-pibrentasvir-g-p/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Glecaprevir/Pibrentasvir solid compositions II
Expiry date: 2036-07-18 The present invention features solid pharmaceutical compositions comprising Compound 1 and Compound 2. In one embodiment, the solid pharmaceutical composition includes (1) a first layer which comprises 100 mg Compound 1, as well as a pharmaceutically acceptable hydrophilic polymer and a pharmaceutically acceptable surfactant, all of which are formulated in amorphous solid dispersion; and (2) a second layer which comprises 40 mg Compound 2, as well as a pharmaceutically acceptable hydrophilic polymer and a pharmaceutically acceptable surfactant, all of which are formulated in amorphous solid dispersion. |
WO2017015211 | Composition | Abbvie Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | South Africa, Mongolia | Australia, Canada, Japan, Korea, Republic of, Israel, New Zealand, Panama |
| Filed | Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro, Serbia, Ecuador, Guatemala, Thailand | Korea, Republic of, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania, Slovenia, New Zealand, Singapore, Hong Kong |
| Not in force | World Intellectual Property Organization (WIPO), Brazil, China, Colombia, Philippines, Peru, Dominican Republic, Turkmenistan, Belarus, Tajikistan, Kazakhstan, Egypt, Indonesia, Viet Nam, India, Mexico, Moldova, Republic of, Malaysia, Ukraine | United States of America, World Intellectual Property Organization (WIPO), Costa Rica, Chile, Russian Federation |
MPP Licence(s)
MPP licence on Glecaprevir/Pibrentasvir (G/P)
https://medicinespatentpool.org/licence-post/glecaprevir-pibrentasvir-g-p/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Glecaprevir/Pibrentasvir solid compositions I
Expiry date: 2036-06-24 The present invention features solid pharmaceutical compositions comprising Compound 1 and Compound 2. In one embodiment, the solid pharmaceutical composition includes (1) a first layer which comprises 100 mg Compound 1, as well as a pharmaceutically acceptable hydrophilic polymer and a pharmaceutically acceptable surfactant, all of which are formulated in amorphous solid dispersion; and (2) a second layer which comprises 40 mg Compound 2, as well as a pharmaceutically acceptable hydrophilic polymer and a pharmaceutically acceptable surfactant, all of which are formulated in amorphous solid dispersion. |
WO2016210273 | Composition | Abbvie Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Mexico, South Africa, Mongolia, Malaysia, Colombia | Australia, Israel, Japan, Korea, Republic of, United States of America, Panama, New Zealand |
| Filed | Brazil, Türkiye, India, Ecuador, Guatemala, Thailand, Albania, North Macedonia, Serbia, Bosnia and Herzegovina, Montenegro | Canada, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, New Zealand, Singapore, Hong Kong, Iceland, Norway, Poland, Romania, San Marino, Croatia, Latvia, Lithuania, Malta, Slovenia |
| Not in force | World Intellectual Property Organization (WIPO), Philippines, China, Dominican Republic, Peru, Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Egypt, Indonesia, Viet Nam, Ukraine | Japan, United States of America, World Intellectual Property Organization (WIPO), Chile, Costa Rica, Russian Federation |
MPP Licence(s)
MPP licence on Glecaprevir/Pibrentasvir (G/P)
https://medicinespatentpool.org/licence-post/glecaprevir-pibrentasvir-g-p/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Glecaprevir crystal forms
Expiry date: 2035-06-05 The present invention features crystalline forms of Compound I. In one embodiment, a crystalline form of Compound I has characteristic peaks in the PXRD pattern as shown in any one of Figures 1-4. |
WO2015188045 | Polymorphs | Abbvie Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Mexico | United States of America, Australia |
| Filed | Türkiye, North Macedonia, Albania, Serbia | Canada, Japan, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania, Slovenia |
| Not in force | World Intellectual Property Organization (WIPO), Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro, Serbia, Morocco, China | Australia, Japan, World Intellectual Property Organization (WIPO), Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania, Slovenia |
MPP Licence(s)
MPP licence on Glecaprevir/Pibrentasvir (G/P)
https://medicinespatentpool.org/licence-post/glecaprevir-pibrentasvir-g-p/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Pibrentasvir crystal forms
Expiry date: 2035-05-08 The present invention features crystalline forms of Compound I. In one embodiment, a crystalline form of Compound I has characteristic peaks in the PXRD pattern as shown in one of Figures 1-10. |
WO2015171993 | Polymorphs | Abbvie Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Mexico | Australia, Japan, United States of America |
| Filed | Albania, Serbia, Türkiye, North Macedonia | Canada, Liechtenstein, Italy, Norway, Malta, Denmark, Belgium, United Kingdom, Greece, Netherlands, Hungary, Croatia, Switzerland, Spain, San Marino, Slovenia, Austria, Romania, Iceland, Cyprus, Finland, France, Bulgaria, Slovakia, Poland, Latvia, Ireland, Estonia, Germany, Luxembourg, Portugal, Czechia, Lithuania, Monaco, Sweden, United States of America |
| Not in force | World Intellectual Property Organization (WIPO), China, Morocco, Albania, Serbia, Bosnia and Herzegovina, Montenegro, Türkiye, North Macedonia, Mexico | World Intellectual Property Organization (WIPO), Australia, Liechtenstein, Italy, Norway, Malta, Denmark, Belgium, United Kingdom, Greece, Netherlands, Hungary, Croatia, Switzerland, Spain, San Marino, Slovenia, Austria, Romania, Iceland, Cyprus, Finland, France, Bulgaria, Slovakia, Poland, Latvia, Ireland, Estonia, Germany, Luxembourg, Portugal, Czechia, Lithuania, Monaco, Sweden, Japan |
MPP Licence(s)
MPP licence on Glecaprevir/Pibrentasvir (G/P)
https://medicinespatentpool.org/licence-post/glecaprevir-pibrentasvir-g-p/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Glecaprevir/Pibrentasvir use in HCV (without IFN or RBV) II
Expiry date: 2035-04-01 The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents to a subject with HCV infection, wherein the treatment lasts for 12 weeks and does not include administration of either interferon or ribavirin, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof. |
WO2015153793 | Use | Abbvie Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Mexico | Australia, Japan, United States of America |
| Filed | China, Albania, North Macedonia, Serbia, Türkiye | Canada, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Finland, Hungary, Iceland, Ireland, Norway, Poland, Portugal, Romania, San Marino, Bulgaria, Croatia, Cyprus, Czechia, Denmark, Estonia, Latvia, Lithuania, Malta, Monaco, Slovakia, Slovenia, Spain |
| Not in force | World Intellectual Property Organization (WIPO), China, Bosnia and Herzegovina, Montenegro, Brazil | Australia, Japan, United States of America, World Intellectual Property Organization (WIPO) |
MPP Licence(s)
MPP licence on Glecaprevir/Pibrentasvir (G/P)
https://medicinespatentpool.org/licence-post/glecaprevir-pibrentasvir-g-p/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Glecaprevir/Pibrentasvir and RBV use in HCV (without IFN) II
Expiry date: 2035-04-01 The present invention features interferon-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents and ribavirin to a subject with HCV infection, wherein the treatment lasts for 12 weeks and does not include administration of interferon, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof. |
WO2015153792 | Use | Abbvie Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | ||
| Filed | Taiwan, Province of China | |
| Not in force | World Intellectual Property Organization (WIPO), China, Mexico, Albania, North Macedonia, Serbia, Türkiye, Bosnia and Herzegovina, Montenegro | Australia, Canada, Japan, United States of America, World Intellectual Property Organization (WIPO), Belgium, Germany, France, Finland, Greece, Hungary, Iceland, Ireland, Italy, Netherlands, Norway, Poland, Portugal, Romania, San Marino, Austria, Bulgaria, Croatia, Cyprus, Czechia, Denmark, Estonia, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, Monaco, Slovakia, Slovenia, Spain, Sweden, Switzerland, United Kingdom |
MPP Licence(s)
MPP licence on Glecaprevir/Pibrentasvir (G/P)
https://medicinespatentpool.org/licence-post/glecaprevir-pibrentasvir-g-p/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Glecaprevir/Pibrentasvir use in HCV (without IFN or RBV)
Expiry date: 2034-03-14 The present invention features interferon- and ribavirin-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents without interferon and ribavirin to a subject with HCV infection, wherein the treatment lasts for 12 weeks, and said at least two direct acting antiviral agents comprise (a) Compound 1 or a pharmaceutically acceptable salt thereof and (b) Compound 2 or a pharmaceutically acceptable salt thereof. |
WO2014152514 | Use | Abbvie Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Brazil, Mexico, Serbia, South Africa, Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Türkiye, North Macedonia, Albania | Canada, Australia, Cyprus, Denmark, Spain, Israel, Japan, Korea, Republic of, New Zealand, Poland, Portugal, Slovenia, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, Russian Federation, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Monaco, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania |
| Filed | Serbia, Türkiye, North Macedonia, Albania | Cyprus, Denmark, Spain, Hong Kong, Korea, Republic of, Poland, Portugal, Singapore, Slovenia, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Monaco, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania |
| Not in force | World Intellectual Property Organization (WIPO), China, Mexico, Serbia, Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro | Cyprus, Denmark, Spain, Japan, Poland, Portugal, Slovenia, Taiwan, Province of China, United States of America, World Intellectual Property Organization (WIPO), Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, Russian Federation, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Monaco, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania |
MPP Licence(s)
MPP licence on Glecaprevir/Pibrentasvir (G/P)
https://medicinespatentpool.org/licence-post/glecaprevir-pibrentasvir-g-p/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Glecaprevir/Pibrentasvir and RBV use in HCV (without IFN)
Expiry date: 2034-03-14 The present invention features interferon -free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the treatment comprises administering at least two direct acting antiviral agents and ribavirin to a subject with HCV infection, wherein the treatment lasts for 12 weeks and does not include administration of interferon, and said at least two direct acting antiviral agents comprise (a) Compound 1 and (b) Compound 2 or a pharmaceutically acceptable salt thereof as disclosed in the description. |
WO2014152635 | Use | Abbvie Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Serbia, South Africa | Israel, Korea, Republic of |
| Filed | Canada, Denmark, Spain, Hong Kong, Croatia, Israel, Poland, Portugal, Singapore, Slovenia, Taiwan, Province of China, Norway, Cyprus, San Marino | |
| Not in force | World Intellectual Property Organization (WIPO), Brazil, China, Mexico, Serbia, Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro | Australia, Denmark, Spain, Hong Kong, Croatia, Japan, New Zealand, Poland, Portugal, Slovenia, Taiwan, Province of China, United States of America, World Intellectual Property Organization (WIPO), Russian Federation, Norway, Cyprus, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Monaco, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Iceland, Malta, San Marino, Romania, Latvia, Lithuania |
MPP Licence(s)
MPP licence on Glecaprevir/Pibrentasvir (G/P)
https://medicinespatentpool.org/licence-post/glecaprevir-pibrentasvir-g-p/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Pibrentasvir use in HCV
Expiry date: 2033-09-17 Pan-genotypic HCV inhibitors are described. This invention also relates to methods of using these inhibitors to treat HCV infection. |
WO2014047039 | Use | Abbvie Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Brazil, Mexico, South Africa, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro, Serbia | Australia, Japan, New Zealand, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania, Slovenia |
| Filed | Türkiye, North Macedonia, Albania, Serbia | Canada, Hong Kong, Singapore, Taiwan, Province of China, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania, Slovenia |
| Not in force | World Intellectual Property Organization (WIPO), China, Mexico, Bosnia and Herzegovina, Montenegro | Japan, United States of America, World Intellectual Property Organization (WIPO), Russian Federation |
MPP Licence(s)
MPP licence on Glecaprevir/Pibrentasvir (G/P)
https://medicinespatentpool.org/licence-post/glecaprevir-pibrentasvir-g-p/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Direct-acting antiviral (DAA) combinations without IFN or RBV
Expiry date: 2032-10-19 The present invention features interferon- and ribavirin-free therapies for the treatment of HCV. Preferably, the treatment is over a shorter duration of treatment, such as no more than 12 weeks. In one aspect, the therapies comprise administering at least two direct acting antiviral agents without interferon and ribavirin to a subject with HCV infection. For example, the therapies comprise administering to a subject an effective amounts of therapeutic agent 1 (ABT) or therapeutic agent 2 (=ABT-333) or therapeutic agent 3 (=ABT-072) or therapeutic agent 4 (ABT), and an inhibitor of cytochrome P450 (e.g., ritonavir). |
WO2013059638 | Combination | Abbvie Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Mexico, Philippines, South Africa | United States of America |
| Filed | Switzerland, Germany, Denmark, Spain, Hong Kong, Israel, Singapore, Taiwan, Province of China, Cyprus | |
| Not in force | Argentina, China, Dominican Republic, Albania, North Macedonia, Serbia, Türkiye, Armenia, Azerbaijan, Belarus, Kyrgyzstan, Kazakhstan, Tajikistan, Turkmenistan, Philippines, World Intellectual Property Organization (WIPO), Brazil, Bosnia and Herzegovina, Montenegro | Canada, Australia, Switzerland, Chile, Germany, Denmark, Spain, United Kingdom, Japan, Portugal, Sweden, Taiwan, Province of China, United States of America, Uruguay, Austria, Belgium, Bulgaria, Cyprus, Czechia, Estonia, Finland, France, Greece, Croatia, Hungary, Ireland, Iceland, Italy, Liechtenstein, Lithuania, Luxembourg, Latvia, Monaco, Malta, Netherlands, Norway, Poland, Romania, Slovenia, Slovakia, San Marino, Russian Federation, New Zealand, World Intellectual Property Organization (WIPO) |
MPP Licence(s)
MPP licence on Glecaprevir/Pibrentasvir (G/P)
https://medicinespatentpool.org/licence-post/glecaprevir-pibrentasvir-g-p/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Pibrentasvir compound II
Expiry date: 2032-02-24 Compounds effective in inhibiting replication of Hepatitis C virus ("HCV") are described. This invention also relates to processes of making such compounds, compositions comprising such compounds, and methods of using such compounds to treat HCV infection. |
WO2012116257 | Compound | Abbvie Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | China, Mexico | Taiwan, Province of China, Spain, Germany, France, United Kingdom, Italy |
| Filed | Spain | |
| Not in force | World Intellectual Property Organization (WIPO), Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro, Serbia | Canada, Japan, United States of America, World Intellectual Property Organization (WIPO), Belgium, Luxembourg, Netherlands, Switzerland, Sweden, Austria, Liechtenstein, Greece, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania, Slovenia |
MPP Licence(s)
MPP licence on Glecaprevir/Pibrentasvir (G/P)
https://medicinespatentpool.org/licence-post/glecaprevir-pibrentasvir-g-p/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Pibrentasvir compound
Expiry date: 2031-10-12 Compounds effective in inhibiting replication of Hepatitis C virus (HCV) are described. This invention also relates to processes of making such compounds, compositions comprising such compounds, and methods of using such compounds to treat HCV infection. |
WO2012051361 | Compound | Abbott Laboratories | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Colombia, Argentina, China, Dominican Republic, Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Moldova, Republic of, Ecuador, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro, Serbia, Mexico, Peru, Ukraine, Bolivia (Plurinational State of), Indonesia, Malaysia, Paraguay, Philippines, Viet Nam, South Africa, Brazil | United States of America, Australia, Chile, Japan, Korea, Republic of, New Zealand, Singapore, Taiwan, Province of China, Uruguay, Denmark, Spain, Portugal, Slovenia, Canada, Israel, Hong Kong, Russian Federation, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Monaco, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, Croatia, Romania, Latvia, Lithuania, Panama |
| Filed | Ecuador, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro, Serbia, India, Pakistan, Paraguay, Thailand, Venezuela (Bolivarian Republic of), Guatemala | Denmark, Spain, Portugal, Slovenia, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Monaco, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania, Bahrain, Kuwait, Qatar, Saudi Arabia, Oman, United Arab Emirates |
| Not in force | World Intellectual Property Organization (WIPO), Argentina, China, Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Moldova, Republic of, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro, Serbia, Mexico, Peru, Egypt, Mongolia, Viet Nam | United States of America, World Intellectual Property Organization (WIPO), Chile, Costa Rica, New Zealand, Uruguay, Denmark, Spain, Portugal, Slovenia, Canada, Russian Federation, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Monaco, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania |
MPP Licence(s)
MPP licence on Glecaprevir/Pibrentasvir (G/P)
https://medicinespatentpool.org/licence-post/glecaprevir-pibrentasvir-g-p/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Glecaprevir compound
Expiry date: 2031-09-20 The present invention discloses compounds of Formula (I) or pharmaceutically acceptable salts, esters, or prodrugs thereof: Formula (I) which inhibit serine protease activity, particularly the activity of hepatitis C virus (HCV) NS3-NS4A protease. Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention. |
WO2012040167 | Compound | Enanta Pharmaceuticals, Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Argentina, Brazil, China, Colombia, Dominican Republic, Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Moldova, Republic of, Ecuador, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro, Serbia, Guatemala, Mexico, Peru, South Africa, India, Bolivia (Plurinational State of), Paraguay, Mongolia, Philippines, Malaysia, Pakistan, Indonesia, Ukraine | Canada, Australia, Cyprus, Denmark, Spain, Hong Kong, Croatia, Israel, Japan, Korea, Republic of, New Zealand, Portugal, Singapore, Slovenia, San Marino, United States of America, Chile, Costa Rica, Russian Federation, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Monaco, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, Romania, Latvia, Lithuania, Uruguay, Panama, Bahrain, Kuwait, Qatar, Saudi Arabia, Oman, United Arab Emirates, Macao |
| Filed | Argentina, Viet Nam, Venezuela (Bolivarian Republic of), Thailand | Cyprus, Denmark, Spain, Croatia, Portugal, Slovenia, San Marino, Taiwan, Province of China, Luxembourg, Netherlands, Hungary, Poland, Norway, Lithuania, Bahrain, Kuwait, Qatar, Saudi Arabia, Oman, United Arab Emirates |
| Not in force | World Intellectual Property Organization (WIPO), Argentina, Colombia, Dominican Republic, Ecuador, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro, Serbia, Guatemala, India, Egypt, Malaysia, Indonesia | Australia, Cyprus, Denmark, Spain, Croatia, Japan, Korea, Republic of, Portugal, Slovenia, San Marino, United States of America, World Intellectual Property Organization (WIPO), Costa Rica, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Monaco, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, Romania, Latvia, Lithuania, Uruguay, Bahrain, Kuwait, Qatar, Saudi Arabia, Oman, United Arab Emirates |
MPP Licence(s)
MPP licence on Glecaprevir/Pibrentasvir (G/P)
https://medicinespatentpool.org/licence-post/glecaprevir-pibrentasvir-g-p/Supporting material
Publications
Thomas DL, Owen A, Kiser JJ. Prospects for Long-Acting Treatments for Hepatitis C. Clin Infect Dis. 2022 Nov 21;75(Suppl 4):S525-S529. doi: 10.1093/cid/ciac715. PMID: 36410380; PMCID: PMC9678383.
In 2019, more than 4 years after the widespread availability of safe, oral, curative treatments, an estimated 58 million people were living with hepatitis C virus infections (PLWHC). Additional tools may enable those not yet reached to be treated. One such tool could be long-acting parenteral formulations of HCV treatments, which may allow PLWHC to be diagnosed and cured in a single encounter. Although existing highly effective oral medications might be formulated as long-acting parenteral treatments, pharmacological, regulatory, patent, and medical challenges have to be overcome; this requires the concerted efforts of PLWHC, researchers, funding agencies, industry, the World Health Organization, and other stakeholders.
Additional documents
No documents were uploaded
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Not provided