
|
Developed by 

|
Supported by 

|
Insulin efsitora
Developer(s)

|
Eli Lilly Originator
https://www.lilly.com/uk/
United States of America Eli Lilly and Company, commonly known as Lilly, is an American multinational pharmaceutical company headquartered in Indianapolis, Indiana. Founded nearly 150 years ago, Lilly focuses on developing medicines that improve lives worldwide. The company is renowned for its contributions in biomedicine and pharmaceuticals, with a diverse portfolio including well-known products like Trulicity, Jardiance |
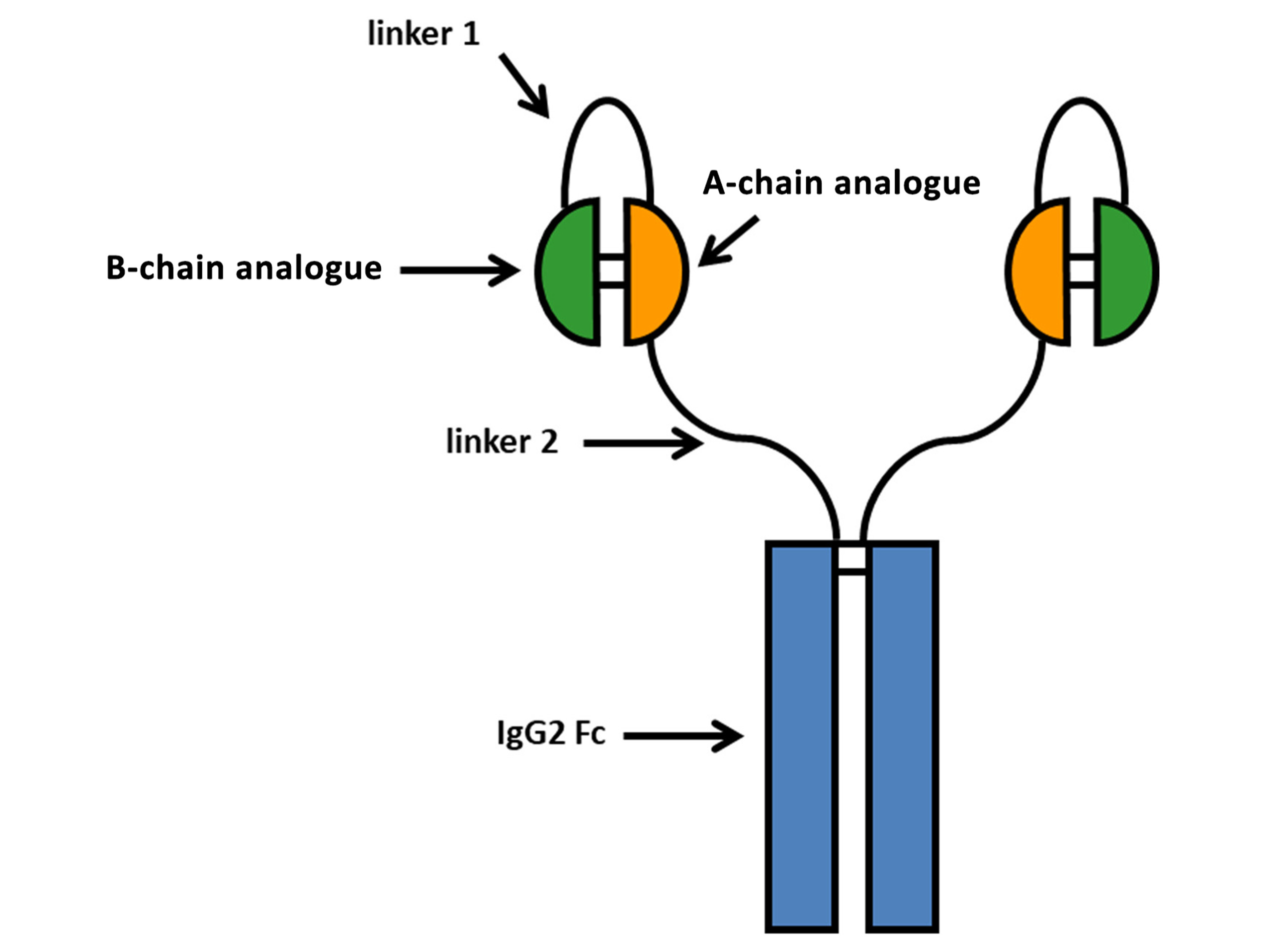
Drug structure

Schematic of weekly basal insulin Fc (BIF) structure
Moyers, J. S., Hansen, R. J., Day, J. W., Dickinson, C. D., Zhang, C., Ruan, X., Ding, L., Brown, R. M., Baker, H. E., & Beals, J. M. (2022). Preclinical Characterization of LY3209590, a Novel Weekly
Drug information
Associated long-acting platforms
Solution
Administration route
Subcutaneous
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
User acceptance
Not provided
Dosage
Available dose and strength
500 units/mL
Frequency of administration
Once weekly
Maximum dose
Not provided
Recommended dosing regimen
Dosage Regimen for Efsitora (500 units/mL): 1. Loading Dose (Week 1—First Injection Only): Administer a one-time loading dose calculated as usual daily basal insulin dose (units) × 7 (rounded to the nearest 10 units) × 3. This loading dose is intended to provide weekly basal coverage. 2. Maintenance Dose (Week 2 Onwards): Administer a weekly dose calculated as usual daily basal insulin dose (units) × 7 (rounded to the nearest 10 units) × 3. This dose should be given once weekly thereafter.
Additional comments
Not provided
Dosage link(s)
Not provided
Drug information
Drug's link(s)
Generic name
Brand name
Compound type
Summary
Approval status
Regulatory authorities
Delivery device(s)
No delivery device
Scale-up and manufacturing prospects
Scale-up prospects
Not provided
Tentative equipment list for manufacturing
1. Bioreactor 2. Seed culture tanks 3. Homogenizer 4. Reaction vessel 5. Stirrer 6. Inert gas supply systems 7. Ultrafiltration units
Manufacturing
1. Recombinant Expression of Insulin in Saccharomyces cerevisiae 2. Purification of insulin analog 3. Site-specific conjugation 4. Purification of the conjugated insulin 5. Excipient addition and formulation preparation
Specific analytical instrument required for characterization of formulation
1. Ion-exchange chromatography/Reverse-phase chromatography/Size-exclusion chromatography 2. High-Performance Liquid Chromatography (HPLC)
Clinical trials
QWINT-1
Identifier
NCT05662332
Link
https://clinicaltrials.gov/study/NCT05662332
Phase
Phase III
Status
Completed
Sponsor
Eli Lilly and Company
More details
The main purpose of this study is to determine the efficacy and safety of insulin efsitora alfa (LY3209590) administered weekly using a fixed dose escalation compared to insulin glargine in adults with type 2 diabetes (T2D) who are starting basal insulin therapy for the first time.
Purpose
A Study of Insulin Efsitora Alfa (LY3209590) Compared to Glargine in Adult Participants With Type 2 Diabetes Who Are Starting Basal Insulin for the First Time (QWINT-1)
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-01-14
Anticipated Date of Last Follow-up
2024-10-28
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2024-07-19
Actual Completion Date
2024-07-19
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Have a diagnosis of T2D according to the World Health Organization criteria. * Have an HbA1c of 7.0% to 10.0%, inclusive, at screening. * Are on a stable treatment of at least 1 antihyperglycemic medication, for at least 3 months prior to screening, and willing to continue the stable treatment for the duration of the study. * Are insulin naive Exceptions: * short-term insulin treatment for a maximum of 14 days, prior to screening, and * prior insulin treatment for gestational diabetes. Exclusion Criteria: * Have a diagnosis of type 1 diabetes (T1D), latent autoimmune diabetes, or specific type of diabetes other than T2D, for example, monogenic diabetes, diseases of the exocrine pancreas, or drug induced or chemical-induced diabetes. * Have a history of \>1 episod
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
795
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
18237
Identifier
NCT05275400
Link
https://clinicaltrials.gov/study/NCT05275400
Phase
Phase III
Status
Completed
Sponsor
Eli Lilly and Company
More details
The reason for this study is to see if the study drug insulin efsitora alfa (LY3209590) is safe and effective in participants with Type 2 diabetes that have already been treated with basal insulin. The study consists of a 3-week screening/lead-in period, a 78-week treatment period and a 5-week safety follow-up period. The study will last up to 86 weeks.
Purpose
A Study of Insulin Efsitora Alfa (LY3209590) Compared With Insulin Degludec in Participants With Type 2 Diabetes Currently Treated With Basal Insulin
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-03-08
Anticipated Date of Last Follow-up
2024-05-24
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2024-05-15
Actual Completion Date
2024-05-15
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Have been diagnosed with Type 2 diabetes according to the World Health Organization (WHO) criteria treated with basal insulin * Are receiving ≥10 units of basal insulin per day and ≤110 units per day at screening * Have HbA1c value of 6.5% - 10% inclusive, at screening * Have a Body mass index (BMI) less than or equal to 45 kilogram/square meter (kg/m²) * Have been treated with one of the following stable insulin regimens at least 90 days prior to screening: * once daily U100 or U200 of insulin degludec * once daily U100 or U300 of insulin glargine * once or twice daily U100 of insulin detemir, or * once or twice daily human insulin NPH * acceptable non insulin glucose lowering therapies may include 0 to up to 3 of the following: * dipeptidyl peptidase (D
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
986
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
QWINT-5
Identifier
NCT05463744
Link
https://clinicaltrials.gov/study/NCT05463744
Phase
Phase III
Status
Completed
Sponsor
Eli Lilly and Company
More details
The main purpose of this study is to measure the safety and efficacy of insulin efsitora alfa (LY3209590) compared with insulin degludec in participants with type 1 diabetes treated with multiple daily injection therapy.
Purpose
A Study of Insulin Efsitora Alfa (LY3209590) Compared With Insulin Degludec in Participants With Type 1 Diabetes Treated With Multiple Daily Injection Therapy
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-08-12
Anticipated Date of Last Follow-up
2024-07-26
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2024-05-07
Actual Completion Date
2024-05-07
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Have a clinical diagnosis of type 1 diabetes for at least 1 year prior to screening * Have received treatment with basal-bolus insulin analog multiple daily injection therapy according to the local product label for at least 90 days prior to screening * Have an HbA1c value of 7.0% to 10.0%, inclusive, as determined by the central laboratory at screening. * Have a body mass index of ≤35 kilogram/square meter (kg/m²) Exclusion Criteria: * Have a diagnosis of type 2 diabetes, latent autoimmune diabetes, or specific types of diabetes other than type 1 diabetes * Have a history of more than 1 episode of severe hypoglycemia, within the 6 months prior to screening. * Have a history of more than 1 episode of diabetic ketoacidosis or hyperosmolar state or coma requiring hos
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
692
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
QWINT-2
Identifier
NCT05362058
Link
https://clinicaltrials.gov/study/NCT05362058
Phase
Phase III
Status
Completed
Sponsor
Eli Lilly and Company
More details
The purpose of this study is to determine the effect and safety of insulin efsitora alfa (LY3209590) compared to degludec in adult participants with type 2 diabetes who are starting basal insulin for the first time. The study consists of a 1-week screening period, a 2-week lead-in period, a 52-week treatment period, and a 5-week safety follow-up period. The study will last up to 60 weeks.
Purpose
A Study of Insulin Efsitora Alfa (LY3209590) Compared to Degludec in Adults With Type 2 Diabetes Who Are Starting Basal Insulin for the First Time
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-06-03
Anticipated Date of Last Follow-up
2025-05-05
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2024-04-10
Actual Completion Date
2024-04-10
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Have diagnosis of Type 2 diabetes (T2D) according to the World Health Organization Criteria * Have an Hemoglobin A1c (HbA1c) of 7.0 percent (%) - 10.5% inclusive, at screening * Are on a stable treatment with 1 to 3 antihyperglycemic medication for at least 3 months prior to screening and willing to continue the stable treatment for the duration of the study * These antihyperglycemic medications are accepted in the study * dipeptidyl peptidase-4 (DPP-4) inhibitors * sodium-glucose cotransporter 2 (SGLT2) inhibitors * biguanides, such as metformin * alpha-glucosidase inhibitors * glucagon-like peptide-1 (GLP-1) receptor agonists, oral or injectable * Sulfonylureas, or * Thiazolidinediones. * Are insulin naïve. Exceptions: * short-term insulin treatmen
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
928
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
QWINT-4
Identifier
NCT05462756
Link
https://clinicaltrials.gov/study/NCT05462756
Phase
Phase III
Status
Completed
Sponsor
Eli Lilly and Company
More details
The reason for this study is to evaluate if the once-weekly study drug insulin efsitora alfa (LY3209590) is safe and effective compared with daily insulin glargine in participants with Type 2 diabetes (T2D) that have already been treated with basal insulin and at least 2 injections per day of prandial insulin. The study consists of a 3-week screening/lead-in period, a 26-week treatment period and a 5-week safety follow-up period. The study will last up to 34 weeks.
Purpose
A Study of Insulin Efsitora Alfa (LY3209590) as a Weekly Basal Insulin Compared to Insulin Glargine in Adult Participants With Type 2 Diabetes on Multiple Daily Injections
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-08-11
Anticipated Date of Last Follow-up
2025-04-10
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2024-02-27
Actual Completion Date
2024-02-27
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Have a diagnosis of T2D according to the world health organization (WHO) criteria, currently treated with basal insulin and at least 2 injections of prandial insulin per day. * Are receiving ≥10 units of total basal insulin per day at screening. * Are receiving ≤2 units/kilogram/day of total daily insulin at screening * Have an HbA1c value of 7.0% to 10%, inclusive, as determined by the central laboratory at screening * Have been treated with a stable regimen of one of the following basal insulins used according to local product label with or without noninsulin diabetes therapy for at least 90 days prior to screening * once daily U-100 or U-200 insulin degludec * once daily U-100 or U-300 insulin glargine * once or twice daily U-100 insulin detemir or * once
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
730
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
Excipients
Proprietary excipients used
No proprietary excipient used
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
No novel excipient or existing excipient used
Residual solvents used
No residual solvent used
Patent info
Description
Methods of treating diabetes
Brief description
Described herein are fixed doses and dosing regimens for long-acting insulin receptor agonists suitable for once-weekly dosing, such as weekly basal insulin-Fc (BIF).
Representative patent
US20240082363A1
Category
Not provided
Patent holder
Eli Lilly and Co
Exclusivity
Not provided
Expiration date
Not provided
Status
Pending
Supporting material
Publications
Wysham, C., Bajaj, H. S., Del Prato, S., Franco, D. R., Kiyosue, A., Dahl, D., Zhou, C., Carr, M. C., Case, M., Firmino Gonçalves, L., & QWINT-2 Investigators (2024). Insulin Efsitora versus Degludec in Type 2 Diabetes without Previous Insulin Treatment. The New England journal of medicine, 391(23), 2201–2211. https://doi.org/10.1056/NEJMoa2403953
Abstract
Background: Insulin efsitora alfa (efsitora) is a new basal insulin designed for once-weekly administration. Data on safety and efficacy have been limited to small, phase 1 or phase 2 trials.
Methods: We conducted a 52-week, phase 3, parallel-design, open-label, treat-to-target trial involving adults with type 2 diabetes who had not previously received insulin. Participants were randomly assigned in a 1:1 ratio to receive efsitora or degludec. The primary end point was the change in the glycated hemoglobin level from baseline to week 52; we hypothesized that efsitora would be noninferior to degludec (noninferiority margin, 0.4 percentage points). Secondary and safety end points included the change in the glycated hemoglobin level in subgroups of participants using and not using glucagon-like peptide-1 (GLP-1) receptor agonists, the percentage of time that the glucose level was in the target range of 70 to 180 mg per deciliter in weeks 48 through 52, and hypoglycemic episodes.
Results: A total of 928 participants underwent randomization (466 to the efsitora group and 462 to the degludec group). The mean glycated hemoglobin level decreased from 8.21% at baseline to 6.97% at week 52 with efsitora (least-squares mean change, -1.26 percentage points) and from 8.24% to 7.05% with degludec (least-squares mean change, -1.17 percentage points) (estimated treatment difference, -0.09 percentage points; 95% confidence interval [CI], -0.22 to 0.04), findings that showed noninferiority. Efsitora was noninferior to degludec with respect to the change in the glycated hemoglobin level in participants using and not using GLP-1 receptor agonists. The percentage of time that the glucose level was within the target range was 64.3% with efsitora and 61.2% with degludec (estimated treatment difference, 3.1 percentage points; 95% CI, 0.1 to 6.1). The rate of combined clinically significant or severe hypoglycemia was 0.58 events per participant-year of exposure with efsitora and 0.45 events per participant-year of exposure with degludec (estimated rate ratio, 1.30; 95% CI, 0.94 to 1.78). No severe hypoglycemia was reported with efsitora; six episodes were reported with degludec. The incidence of adverse events was similar in the two groups.
Conclusions: In adults with type 2 diabetes who had not previously received insulin, once-weekly efsitora was noninferior to once-daily degludec in reducing glycated hemoglobin levels. (Funded by Eli Lilly; QWINT-2 ClinicalTrials.gov number, NCT05362058.).
Bergenstal, R. M., Philis-Tsimikas, A., Wysham, C., Carr, M. C., Bue-Valleskey, J. M., Botros, F. T., Blevins, T., & Rosenstock, J. (2024). Once-weekly insulin efsitora alfa: Design and rationale for the QWINT phase 3 clinical development programme. Diabetes, obesity & metabolism, 26(8), 3020–3030. https://doi.org/10.1111/dom.15604
Aims: Insulin efsitora alfa (efsitora) is a once-weekly basal insulin. This review describes the study design and rationale of the efsitora phase 3 Once Weekly (QW) Insulin Therapy (QWINT) clinical development programme, including the five trials, QWINT-1 through QWINT-5.
Materials and methods: The five trials included insulin-naïve adults (QWINT-1 and -2) with type 2 diabetes (T2D), adults with T2D previously treated with basal insulin (QWINT-3 and -4), and QWINT-5 in adults with type 1 diabetes. All five trials were designed as multicentre, randomized, controlled, open-label, treat-to-target studies to investigate the efficacy and safety of efsitora versus active once-daily basal insulin comparators (insulin glargine U100 or insulin degludec U100). The primary objective of each trial is to compare the change in HbA1c from baseline to week 26 or 52 between efsitora and the active comparator. The key secondary objectives include change in fasting glucose, insulin dose and continuous glucose monitoring variables, and patient-reported outcome questionnaires.
Conclusions: The QWINT development programme includes a racially and geographically diverse population to provide important information regarding the efficacy and safety of efsitora and its clinical management of people with diabetes.
Additional documents
No documents were uploaded
Useful links
There are no additional links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Not provided