
|
Developed by 

|
Supported by 

|
Insulin icodec
Developer(s)

|
Novo Nordisk Originator
https://www.novonordisk.com/science-and-technology/r-d-pipeline.html
Denmark Novo Nordisk is a global healthcare company specializing in diabetes care. Founded in Denmark in 1923, it is renowned for its leadership in insulin production and diabetes treatments. Novo Nordisk's product portfolio includes insulin analogs, GLP-1 receptor agonists, and other pharmaceuticals for managing diabetes and other chronic conditions. |
Drug structure

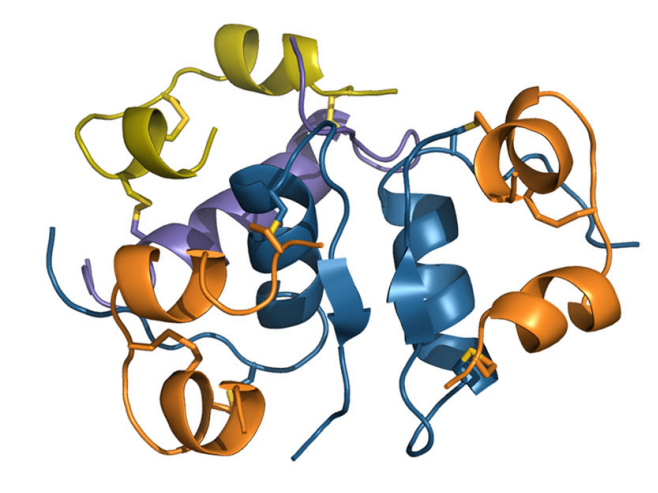
3D Trimeric Arrangement of insulin icodec in the crystal
Hubálek, F., Cramer, C. N., Helleberg, H., Johansson, E., Nishimura, E., Schluckebier, G., Steensgaard, D. B., Sturis, J., & Kjeldsen, T. B. (2024). Enhanced disulphide bond stability contributes to t
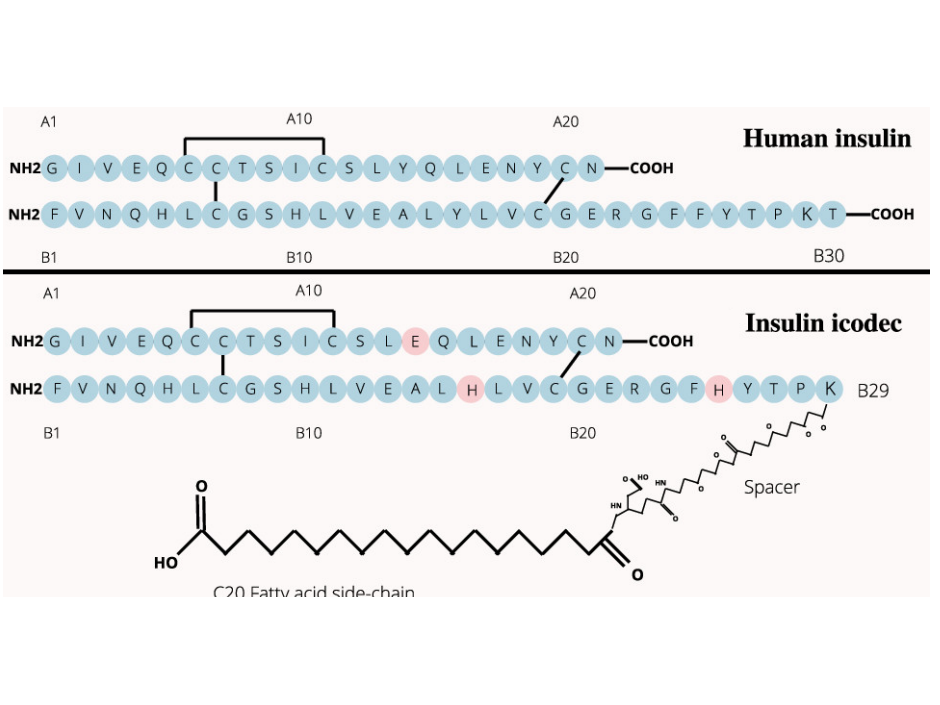
2D structural difference between Insulin and Insulin Icodec
Kandhasamy, R. (2024). Designing insulin analogues with lower binding affinity to insulin receptor than that of insulin icodec [Preprint]. Preprints. https://doi.org/10.20944/preprints202404.1922.v1
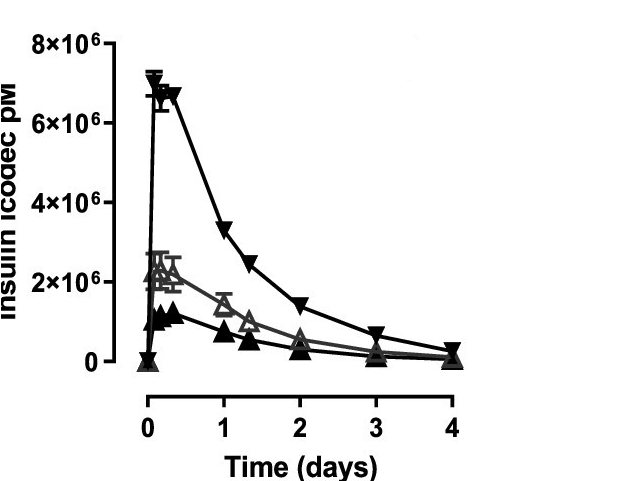
Insulin icodec concentration Vs time curve
Kjeldsen, T. B., Hubálek, F., Hjørringgaard, C. U., Tagmose, T. M., Nishimura, E., Stidsen, C. E., Porsgaard, T., Fledelius, C., Refsgaard, H. H. F., Gram-Nielsen, S., Naver, H., Pridal, L., Hoeg-Jens
Drug information
Associated long-acting platforms
Solution
Administration route
Subcutaneous
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
User acceptance
The reduced injection frequency of once-weekly insulin icodec has been linked to better adherence and acceptance among patients. Studies suggest that the lower injection burden could facilitate greater willingness to initiate or continue insulin therapy, particularly for individuals who find daily injections challenging. In ONWARDS2, T2DM patients using insulin icodec reported significantly higher satisfaction scores compared to those using daily basal insulins. In contrast, patient satisfaction was lower for insulin icodec compared to insulin degludec in type 1 diabetes (ONWARDS 6 trial).
Dosage
Available dose and strength
700 units/1mL; 1050 units/1.5mL & 2100 units/3mL
Frequency of administration
Once weekly
Maximum dose
700 units per SC administration
Recommended dosing regimen
For Type 1 Diabetes Mellitus—insulin icodec + bolus insulin combination therapy. For Type 2 Diabetes Mellitus —initial dose is 70 units, monotherapy, or combination therapy with sulfonylurea/GLP-1 agonist. For patients transitioning to Awiqli once-weekly insulin, both T1DM & T2DM—the recommended dose depends on their previous total daily dose of once- or twice-daily basal insulin. The dosage regimen is as follows: If the previous daily dose was 10 units, give 110 units of Awiqli in week 1 and 110 units in week 2. If the previous daily dose was 11 units, give 70 units of Awiqli in week 1 and 120 units in week 2. If the previous daily dose was 12 units, give 80 units of Awiqli in week 1 and 130 units in week 2. If the previous daily dose was 14 units, give 90 units of Awiqli in week 1.
Additional comments
The prefilled injection has a dose range of 10-700 units/injection (increase the dose by 10 units) No proprietary device is required to deliver insulin icodec. It is administered subcutaneously using a pre-filled pen injector, which is similar in design and technique to those used for other insulins. The pen contains a high-concentration formulation of 700 units/mL, ensuring that the injection volume remains comparable to once-daily basal insulin injections
Dosage link(s)
Drug information
Drug's link(s)
Generic name
Brand name
Compound type
Summary
Approval status
Regulatory authorities
Delivery device(s)
No delivery device
Scale-up and manufacturing prospects
Scale-up prospects
In 2024, Novo Nordisk has invested 4.1 billion to expand the manufacturing capacity and increase the supply of AWQLI. It has five strategic production sites located in Denmark, the USA, France, Brazil, and China.
Tentative equipment list for manufacturing
Peptide synthesizers, mixers, pH meters, and sterile filtration units. No proprietary device is required to deliver insulin icodec. It is administered subcutaneously using a pre-filled pen injector, which is similar in design and technique to those used for other insulins.
Manufacturing
Manufacturing of Insulin icodec (Schedule D) follows ICH G7 guidelines. The manufacturing process includes: 1. Insulin icodec (Awiqli) is made using recombinant DNA technology in S. cerevisiae. 2. Drug substance steps: cell expansion, fermentation, recovery, concentration, and purification (incl. enzymatic/chemical steps). 3. Drug product: solution prep, mixing, pH/volume adjust, sterile filtration, aseptic fill into cartridges. 4. Final: capped, inspected, assembled, labelled, stored at 2–8°C. 5. Processes & controls are validated and acceptable.
Specific analytical instrument required for characterization of formulation
1. High-Performance Liquid Chromatography (HPLC) 2. Mass Spectrometry (MS) and LC-MS (Liquid Chromatography-Mass Spectrometry) 3. Insulin Assay Kits or Immunoassays
Clinical trials
NN1436-4571
Identifier
NCT04857398
Link
https://clinicaltrials.gov/study/NCT04857398
Phase
Phase I
Status
Completed
Sponsor
Novo Nordisk A/S
More details
This study is looking at the way insulin icodec stays and moves over time in the blood after injections in Chinese people with type 2 diabetes. Participants will get insulin icodec once a week for 6 weeks. The medicine will be injected under the skin of the thigh. There will also be a run-in period that will last between 1 week and 8 weeks with daily doses of insulin degludec before start on insulin icodec. The study will last for about 15 to 22 weeks. Participants will have about 17 visits with the study doctor including phone contact during your run-in period. Participants will have blood samples taken at the clinic visits. Several samples of participants blood will be taken for up to 48 hours after getting the first and the last dose of insulin icodec. Participants must be a Chinese
Purpose
A Study to Test How a New Long-acting Insulin (Insulin Icodec) Works in the Body of People From China With Type 2 Diabetes
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-04-28
Anticipated Date of Last Follow-up
2023-05-16
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-04-06
Actual Completion Date
2022-04-06
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Chinese male or female * Aged 18-64 years (both inclusive) at the time of signing informed consent * Body mass index between 18 and 38 kg/m\^2 (both inclusive) * HbA1c (glycated haemoglobin) below or equal to 9% at screening * Current daily basal insulin treatment greater than or equal to 0.2 (I)U/kg/day greater than or equal to 60 days prior to the day of screening with or without any of the following anti-diabetic drugs/regimens with stable doses greater than or equal to 60 days prior to the day of screening: * Any metformin formulation * Other oral antidiabetic drugs: DPP-4 (Dipeptidyl-peptidase-4) Inhibitors , SGLT2 (Sodium-glucose co-transporter-2) inhibitors, Oral combination products (for the allowed individual oral antidiabetic drugs) * Oral or injectable GLP
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
24
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
NN1436-4572
Identifier
NCT04582448
Link
https://clinicaltrials.gov/study/NCT04582448
Phase
Phase I
Status
Completed
Sponsor
Novo Nordisk A/S
More details
This study is comparing the concentration of a single dose of insulin icodec when administered in the belly, upper arm and thigh on different occasions. Participants will receive one injection of insulin icodec on three different occasions, each time injected at a different site, i.e. either on our belly, upper arm or thigh. The study will last for about 34 weeks. Participants will have 23 visits with the study doctor. Informed Consent (V0) visit and screening visit (V1) will be performed on two different days. The informed consent visit may be performed via telephone to minimize personal contact with site staff during the coronavirus outbreak. Women cannot take part if pregnant, breast- feeding or plan to become pregnant during the study period.
Purpose
A Study Looking at How Insulin Icodec is Taken up in the Blood When Administered in Different Injection Sites in People With Type 2 Diabetes
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2020-10-01
Anticipated Date of Last Follow-up
2023-01-19
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2021-09-27
Actual Completion Date
2021-09-27
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female * Aged 18-69 years (both inclusive) at the time of signing informed consent * Body mass index between 18.5 and 38.0 kg/m\^2 (both inclusive) * Diagnosed with type 2 diabetes mellitus above or equal to 180 days prior to the day of screening * HbA1c (glycated haemoglobin) below or equal to 9.0 percentage at screening * Current daily basal insulin treatment of 0.2-1.0 (I)U/kg/day (both inclusive) with or without any of the following anti-diabetic drugs/regimens with stable doses above or equal to 90 days prior to the day of screening: * Any metformin formulation * Other oral antidiabetic drugs: DPP-4 inhibitors / SGLT2 inhibitors / Oral combination products (for the allowed individual oral antidiabetic drugs) * Oral or injectable GLP-1 (glucagon-like pept
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
25
Allocation
Randomized
Intervention model
Cross-over assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
NN1436-4569
Identifier
NCT04582435
Link
https://clinicaltrials.gov/study/NCT04582435
Phase
Phase I
Status
Completed
Sponsor
Novo Nordisk A/S
More details
The aim of the study is to improve clinical outcomes for patients with type 2 diabetes by limiting the burden associated with insulin treatment. Participants will get insulin degludec as well as insulin icodec - Insulin icodec is a new medicine while insulin degludec is commonly used and prescribed by doctors. Participants will administer subcutaneous injections of insulin degludec once daily for at least one week (7 injections) but this period may be extended up to 8 weeks. Thereafter, once weekly subcutaneous injections of insulin icodec will follow, resulting in a total of at least 8 but not more than 16 subcutaneous injections of icodec. The study will last for about 17-32 weeks Participants will have at least 5 in-house visits (where participants will stay at the clinic) and 17 out
Purpose
A Trial Investigating the Pharmacokinetic and Pharmacodynamic Properties of Insulin Icodec in Subjects With Type 2 Diabetes
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2020-10-16
Anticipated Date of Last Follow-up
2023-09-14
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-04-22
Actual Completion Date
2022-04-22
Studied populations
Age Cohort
- Children
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female * Aged 18-75 years (both inclusive) at the time of signing informed consent * Body mass index between 18.0 and 38.0 kg/m\^2 (both inclusive) * HbA1c (glycated haemoglobin) below or equal to 9 percentage (75 mmol/mol) at screening * Current daily basal insulin treatment greater than or equal to 0.2 (I)U/kg/day with or without any of the following anti-diabetic drugs/regimens with stable doses greater than or equal to 90 days prior to the day of screening: 1) Any metformin formulation 2) Other oral antidiabetic drugs: DPP-4 nhibitors / SGLT2 inhibitors / Oral combination products (for the allowed individual oral antidiabetic drugs) * Oral or injectable GLP-1 Receptor Agonists Exclusion Criteria: * Known or suspected hypersensitivity to trial product(s)
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
46
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
NN1436-4888
Identifier
NCT05790681
Link
https://clinicaltrials.gov/study/NCT05790681
Phase
Phase I
Status
Completed
Sponsor
Novo Nordisk A/S
More details
Insulin icodec is a new medicine which is under development for use in humans and is not yet available at the pharmacy. It is being developed for the treatment of diabetes, a condition that causes high blood sugar levels. Insulin icodec will be investigated in participants with type 2 diabetes. Participant will get one dose of insulin icodec, which will be administered in the afternoon or evening of the day of dosing. The study will last for about 8 weeks. Insulin icodec will be injected into a skin fold with a small needle (subcutaneous application) using a pen injector prefilled with a volume of 3 milliliter (mL) (a little less than a quarter of a teaspoonful). The amount of insulin icodec participant will receive depends on participant's body weight. Participant must not participate if
Purpose
A Study to Test How New Long-acting Insulin (Insulin Icodec) Works in the Body of Children and Teenagers
Interventions
Not provided
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-04-25
Anticipated Date of Last Follow-up
2024-04-17
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2024-02-08
Actual Completion Date
2024-02-08
Studied populations
Age Cohort
- Children
- Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female * Aged 10 to less than (\<) 18 years at the time of signing informed consent * Diagnosed with type 2 diabetes mellitus greater than or equal to (\>=) 30 days prior to the day of screening * Glycated haemoglobin (HbA1c) less than or equal to (\<=) 10% (86 millimoles per mole \[mmol/mol\]) at screening * Treated with basal insulin, premix insulin or continuous subcutaneous insulin infusion (CSII) with or without bolus insulin or additional anti-diabetic drug(s). * Current daily basal insulin treatment \>= 0.2 (I) units per kilogram per day (U/kg/day) with stable doses \>=30 days prior to the day of screening Exclusion Criteria: * Known or suspected hypersensitivity to study interventions or related products * Female who is pregnant, breast-feeding or i
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
18
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
NN1436-4570
Identifier
NCT04597697
Link
https://clinicaltrials.gov/study/NCT04597697
Phase
Phase I
Status
Completed
Sponsor
Novo Nordisk A/S
More details
Participants will receive one insulin icodec dose, which will be administered in the morning of the day of dosing. The study will last for about 8 weeks. Participants will have 8 visits with the study doctor in the clinical research unit. Insulin icodec will be injected into a skin fold with a small needle (subcutaneous application) using a pen injector prefilled with a volume of 3 mL (about a spoonful). Participants must not participate if they meet certain conditions called exclusion criteria, such as an age of below 18 years or above 70 years, if participants are over- or underweight, using certain medicines, or have serious health conditions (other than impaired liver function ). Women cannot take part if pregnant, breast-feeding or planning to become pregnant during the study peri
Purpose
A Study to Test How a New Long-acting Insulin (Insulin Icodec) Works in the Body of People With Liver Disease
Interventions
Not provided
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2020-12-22
Anticipated Date of Last Follow-up
2024-07-11
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-03-24
Actual Completion Date
2022-03-24
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female * Aged 18-70 years (both inclusive) at the time of signing informed consent * Body mass index between 18.5 and 39.9 kg/m\^2 (both inclusive) Specific inclusion criterion only for subjects with hepatic impairment * Subjects with stable hepatic impairment classified as Child-Pugh grade A, B or C as assessed by the investigator. Stable hepatic impairment is defined as no clinically significant change in disease status, as judged by the investigator. Exclusion Criteria: * Known or suspected hypersensitivity to trial product or related products * Female who is pregnant, breast-feeding or intends to become pregnant or is of child-bearing potential and not using an adequate contraceptive method (adequate contraceptive measures as required by local regulatio
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
25
Allocation
Not provided
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
NN1436-7615
Identifier
NCT06288412
Link
https://clinicaltrials.gov/study/NCT06288412
Phase
Phase I
Status
Not provided
Sponsor
Novo Nordisk A/S
More details
The study will investigate the safety of once weekly insulin icodec subcutaenously (s.c.) during and after exercise and prolonged fasting in patients with type 2 diabetes (T2D). Participants will first receive insulin decludec (Tresiba®, a long-acting insulin taken once daily) for atleast one week. Afterwards participants will receive insulin icodec that will be administered once weekly at the study site (for a minimum of 7 weeks and maximum of 14 weeks). Insulin icodec is a novel long-acting insulin analogue for once-weekly administration for the treatment of type 2 diabetes. The study will last for about 16-30 weeks. Participant must not participate if participant have suspected hypersensitivity reactions to the study products or cardiovascular diseases within the last 180 days. Female p
Purpose
A Study to Test How a New Long-acting Insulin Works in the Body of Patients With Type 2 Diabetes During Exercise and Prolonged Fasting
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2024-02-26
Anticipated Date of Last Follow-up
2025-03-20
Estimated Primary Completion Date
Not provided
Estimated Completion Date
2025-04-14
Actual Primary Completion Date
2025-03-10
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Informed consent obtained before any study-related activities. Study-related activities are any procedures that are carried out as part of the study, including activities to determine suitability for the study. * Male or female. * Age 18-75 years (both inclusive) at the time of signing the informed consent. * Body mass index between 18.0 and 38.0 kilogram per meter\^2 (kg/m\^2) (both inclusive). * Glycated hemoglobin (HbA1c) less than or equal to (\<=) 9 percent (75 millimoles per mole \[mmol/mol\]) at screening. * Treated with basal insulin with or without any of the following anti-diabetic drugs/regimens with stable doses \>= 90 days prior to the day of screening: * Metformin, * Dipeptidyl peptidase 4 (DPP-4) inhibitors, * Sodium-Glucose Transport Protein 2
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
30
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
NN1436-4226
Identifier
NCT03723785
Link
https://clinicaltrials.gov/study/NCT03723785
Phase
Phase I
Status
Completed
Sponsor
Novo Nordisk A/S
More details
This study will be conducted to look at the effect of decreased kidney function when getting one dose of insulin 287 and to guide dosing recommendations in people who have altered kidney function. Insulin 287 works in the body for a long time (long-acting). It is taken once a day by injecting under the skin. The main target patient group for insulin 287 is people with type 2 diabetes. Participants will get just one injection. The study will last for up to 80 days. Participants will have 11 out-patient visits with the study doctor and one in-house visit of 3 days and 2 nights. Participants will have some assessments like several blood draws, electrocardiograms (ECGs), urine collections and capillary blood sugar tests. Participants cannot take part if they are hypersensitive to the study med
Purpose
A Research Study of How a New Medicine NNC0148-0287 C (Insulin 287-aka icodec) Works in the Body of People With Kidney Problems
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2018-11-09
Anticipated Date of Last Follow-up
2024-07-11
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2019-09-06
Actual Completion Date
2019-09-06
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: * Male or female, aged 18-75 years (both inclusive) at the time of signing informed consent. * Body mass index between 18.5 and 32.0 kg/sqm (both inclusive). * Meeting the pre-defined Glomerular Filtration Rate (GFR) values based on a measured GFR using an exogenous substance as tracer (renal group 1-4) or being in treatment with haemodialysis (renal group 5). Exclusion Criteria: * Known or suspected hypersensitivity to trial product or related products. * Impaired liver function defined as Alanine Aminotransferase (ALT) greater than or equal to 2.5 times or Bilirubin greater than 1.5 times upper limit of normal at screening. * Drugs known to affect creatinine clearance including cephalosporin and aminoglycoside antibiotics, flucytosine, cisplatin, cimetidine and tri
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
58
Allocation
Not provided
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
NN1436-4462
Identifier
NCT03945656
Link
https://clinicaltrials.gov/study/NCT03945656
Phase
Phase I
Status
Completed
Sponsor
Novo Nordisk A/S
More details
This study is comparing the effect of a long-acting insulin analogue (insulin 287) with insulin glargine (Lantus®) in subjects with type 2 diabetes. In addition, the study is looking at symptoms of low blood sugar, awareness of low blood sugar and the time and amount of glucose needed to recover from low blood sugar after injecting 2 and 3 times the basal dose of insulin 287 and glargine. The purpose of the study is to make a once-weekly injectable basal insulin treatment for people with type 2 diabetes. Participants will get insulin 287 as well as insulin glargine - which treatment any participant gets first is decided by chance. Insulin 287 is a new medicine; insulin glargine can already be prescribed. The study medicines will be in a pen, and must be injected with a needle in the thigh
Purpose
A Research Study of How Overdosing of a New Once Weekly Medicine NNC0148-0287 C (Insulin 287) Influences the Blood Sugar Level in People With Type 2 Diabetes
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-05-07
Anticipated Date of Last Follow-up
2023-09-01
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2021-09-27
Actual Completion Date
2021-09-27
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female, aged between 18 and 72 years (both inclusive) at the time of signing informed consent. * Body mass index between 18.5 and 37.9 kg/m\^2 (both inclusive). * Diagnosed with type 2 diabetes mellitus greater than or equal to 180 days prior to the day of screening. * Glycosylated haemoglobin type A1c (HbA1c) less than or equal to 9.0% (less than or equal to 74 mmol/mol) at screening. * Current total daily insulin treatment between 0.2 and 1.0 U/kg/day (both inclusive). Exclusion Criteria: * Known or suspected hypersensitivity to trial products or related products. * Female who is pregnant, breast-feeding or intends to become pregnant or is of child-bearing potential and not using an adequate contraceptive method. * Presence or history of any clinically re
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
43
Allocation
Randomized
Intervention model
Cross-over assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
NN1436-4422
Identifier
NCT03766854
Link
https://clinicaltrials.gov/study/NCT03766854
Phase
Phase I
Status
Completed
Sponsor
Novo Nordisk A/S
More details
This study will look at how insulin 287 works, if it is safe and the side effects in people who are Japanese with type 1 diabetes. The study will test how insulin goes through your blood, how long it stays there and how the blood sugar is lowered. Insulin 287 is a new medicine. Insulin glargine is already approved to treat diabetes. The study doctors can prescribe insulin glargine. The participants will get both of the insulins in a random order. The participants will get 8 weekly doses of insulin 287 and 14 daily doses of insulin glargine. There will also be a run-in period of 2 days to 7 weeks when the participants inject insulin glargine every day before they start insulin 287 period or insulin glargine period. All doses will be injected under the skin. During the run-in period, the par
Purpose
A Research Study of How Different Amounts of a New Medicine NNC0148-0287 C (Insulin 287-AKA icodec) Works on the Blood Sugar of People Who Are Japanese With Type 1 Diabetes When Given Once a Week
Interventions
Not provided
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2018-12-07
Anticipated Date of Last Follow-up
2021-03-05
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2019-12-09
Actual Completion Date
2019-12-09
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female, Japanese subjects, aged 20 - 64 years (both inclusive) at the time of signing informed consent. * Diagnosed with type 1 diabetes mellitus greater than or equal to 1 year prior to the day of screening. * Current daily basal insulin treatment greater than or equal to 0.2 U/kg/day. * Body mass index between 18.5 and 28.0 kg/m\^2 (both inclusive). * HbA1c less than or equal to 9.0%. Exclusion Criteria: * History or presence of any clinically relevant respiratory, metabolic, renal, hepatic, gastrointestinal or endocrinological conditions (except conditions associated with diabetes mellitus). * Female who is pregnant, breast-feeding or intends to become pregnant or is of child-bearing potential and not using adequate contraceptive methods. * Known or susp
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
24
Allocation
Randomized
Intervention model
Cross-over assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
NN1436-4225
Identifier
NCT03723772
Link
https://clinicaltrials.gov/study/NCT03723772
Phase
Phase I
Status
Completed
Sponsor
Novo Nordisk A/S
More details
This study compares the new long-acting insulin 287 with the marketed insulin glargine for use in type 1 diabetes. The study will test how insulin is taken up in your blood, how long it stays there and how the blood sugar is lowered. The participant will get both of the insulins in a random order. Insulin 287 is a new medicine while insulin glargine is already approved for the treatment of diabetes and can be prescribed by a doctor. The participant will get 8 weekly doses of insulin 287 and 14 daily doses of insulin glargine. There will also be a run-in period of 2 days to 4 weeks with daily doses of insulin glargine before you start the insulin 287 period. All doses will be injected under the skin. The study will last for about 16 to 24 weeks. The participant will have 27 visits with the
Purpose
A Research Study of How Different Doses of a New Medicine NNC0148-0287 C (Insulin 287) Work on the Blood Sugar in People With Type 1 Diabetes When it is Taken Once a Week
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2018-11-29
Anticipated Date of Last Follow-up
2025-03-10
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2020-06-26
Actual Completion Date
2020-06-26
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female, aged 18-64 years (both inclusive) at the time of signing informed consent * Diagnosed with type 1 diabetes mellitus greater than or equal to 1 year prior to the day of screening * Current daily basal insulin treatment greater than or equal to 0.2 U/kg/day * Body mass index between 18.5 and 29.0 kg/m\^2 (both inclusive) * HbA1c less than or equal to 9.0% Exclusion Criteria: * History or presence of any clinically relevant respiratory, metabolic, renal, hepatic, gastrointestinal or endocrinological conditions. Subjects with complications associated to diabetes can be included only if they are judged to be mild by the investigator. Subjects with other comorbidities (e.g. dyslipidaemia, hypertension and hypothyroidism) can be included if they have a sta
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
66
Allocation
Randomized
Intervention model
Cross-over assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
NN1535-4710
Identifier
NCT05435677
Link
https://clinicaltrials.gov/study/NCT05435677
Phase
Phase I
Status
Completed
Sponsor
Novo Nordisk A/S
More details
This study will look at a new medicine, called IcoSema, for treatment of type 2 diabetes. IcoSema is a combination of a new insulin, called insulin icodec, and a GLP-1 receptor analogue, called semaglutide. Insulin icodec is a possible new medicine. That means that the medicine has not yet been approved by the authorities. Semaglutide is a medicine already approved by the authorities in the EU, USA, China and Japan. The study will look at the way insulin icodec and semaglutide reach and stay in participants blood after injection when given together as IcoSema or alone as insulin icodec and semaglutide. Participants will get each of the 3 medicines (IcoSema, insulin icodec and semaglutide) at 3 different timepoints: The order in which participants get them is decided by chance. Partici
Purpose
A Research Study to Look at How Insulin Icodec and Semaglutide Work in the Body of People From China With Type 2 Diabetes When Given Alone or Together
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-06-22
Anticipated Date of Last Follow-up
2025-03-20
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2023-04-25
Actual Completion Date
2023-04-25
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Chinese male or female * Aged 18-64 years (both inclusive) at the time of signing informed consent * Diagnosed with type 2 diabetes mellitus greater than or equal to 180 days prior to the day of screening * Body mass index between 18.5 and 34.9 kg/m\^2 (both inclusive) * Body weight greater than or equal to 50 kg * HbA1c (glycated haemoglobin) below or equal to 9.0% (75 mmol/mol) * Insulin naïve. However, short-term insulin treatment for a maximum of 14 days prior to the day of screening is allowed, as is prior insulin treatment for gestational diabetes * Stable daily dose(s) including any of the following anti-diabetic drug(s)/regimen within 45 days prior to the day of screening: * Any metformin formulation * DPP-4 (dipeptidyl peptidase-4) inhibitors (participa
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
20
Allocation
Randomized
Intervention model
Cross-over assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
NN1436-4057
Identifier
NCT02148861
Link
https://clinicaltrials.gov/study/NCT02148861
Phase
Phase I
Status
Completed
Sponsor
Novo Nordisk A/S
More details
This trial is conducted in Europe. The aim of the trial is to investigate the safety, tolerability, pharmacokinetics (the exposure of the trial drug in the body) and pharmacodynamics (the effect of the investigated drug on the body) of subcutaneously administered NNC0148-0287 (insulin 287) in subjects with type 2 diabetes
Purpose
A Trial Investigating the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Subcutaneously Administered NNC0148-0287 (Insulin 287) in Subjects With Type 2 Diabetes
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2014-05-26
Anticipated Date of Last Follow-up
2021-03-05
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2015-06-01
Actual Completion Date
2015-06-01
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female, age between 18 and 64 years (both inclusive) at the time of signing informed consent * Females of no childbearing potential \[if surgically sterilized (i.e. tubal ligation, bilateral oophorectomises or hysterectomised) for at least 3 months or if post-menopausal (i.e. as defined by amenorrhoea for at least 12 months prior to screening and documented by FSH (follicle-stimulating hormone) levels above 40 U/L\] * Body mass index (BMI) between 20.0 and 35.0 kg/m\^2 (both inclusive) * Type 2 diabetes mellitus (as diagnosed clinically) for at least 12 months Exclusion Criteria: * Known or suspected hypersensitivity to trial products or related products * Receipt of any investigational medicinal products within 3 months before screening * Use of oral antid
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
49
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Double-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
NN1436-4314
Identifier
NCT02964104
Link
https://clinicaltrials.gov/study/NCT02964104
Phase
Phase I
Status
Completed
Sponsor
Novo Nordisk A/S
More details
This trial is conducted in Europe. The aim of the trial is to investigate the safety, tolerability, pharmacokinetics (the exposure of the trial drug in the body) and pharmacodynamics (the effect of the investigated drug on the body) of insulin 287 in subjects with type 2 diabetes.
Purpose
A Trial Investigating the Safety, Tolerability, Pharmacokinetics (the Exposure of the Trial Drug in the Body) and Pharmacodynamics (the Effect of the Investigated Drug on the Body) of Insulin 287 in S
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2016-11-15
Anticipated Date of Last Follow-up
2021-08-23
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2017-12-12
Actual Completion Date
2017-12-12
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female, age between 18 and 64 years (both inclusive) at the time of signing informed consent. * Subject who is considered to be generally healthy (with the exception of conditions associated with diabetes mellitus), based on the medical history, physical examination, and the results of vital signs, ECG and laboratory safety tests, as judged by the investigator. * Body mass index between 20.0 and 34.9 kg/m\^2 (both inclusive). * Type 2 diabetes mellitus (as diagnosed clinically) for ≥12 months (365 days). * No change in insulin treatment regimen during the last 90 days prior to screening. * Current total daily insulin treatment between 0.3 and 1.0 (I) U/kg/day (both inclusive). Exclusion Criteria: * Known or suspected hypersensitivity to trial products or re
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
50
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Double-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
NN1436-4465
Identifier
NCT03951805
Link
https://clinicaltrials.gov/study/NCT03951805
Phase
Phase II
Status
Completed
Sponsor
Novo Nordisk A/S
More details
This study compares insulin 287 (a possible new medicine) to insulin glargine (a medicine doctors can already prescribe) in people with type 2 diabetes. Different ways of changing the dose of insulin 287 are also compared. This is done to find the best way to change the dose of insulin 287. Participants will either get insulin 287 that they will have to inject once a week or insulin glargine that participants will have to inject once a day. Which treatment participants get is decided by chance. The study will last for about 5 months (23 weeks). Participants will have 14 clinic visits and 6 phone calls with the study doctor. At 3 of the clinic visits participants will be asked not to eat or drink anything (except for water) in the last 8 hours before the visit. During the study, the study d
Purpose
A Research Study to Compare Two Types of Insulin: Insulin 287 and Insulin Glargine in People With Type 2 Diabetes Who Have Not Used Insulin Before
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-05-09
Anticipated Date of Last Follow-up
2021-03-05
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2019-12-12
Actual Completion Date
2020-01-17
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female, aged 18-75 years (both inclusive) at the time of signing informed consent * Diagnosed with type 2 diabetes mellitus greater than or equal to 180 days prior to the day of screening * HbA1c of 7.0-10.0% (53.0-85.8 mmol/mol) (both inclusive) as assessed by central laboratory * Stable daily dose(s) for 90 days prior to the day of screening of any of the following antidiabetic drug(s) or combination regime(s): 1. Any metformin formulations greater than or equal to 1500 mg or maximum tolerated or effective dose (as documented in subject's medical records) 2. Free or fixed combination therapy: Metformin as outlined above plus/minus DPP4i with or without SGLT2i is allowed: i) DPP4i (greater than or equal to half of the maximum approved dose according
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
205
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
NN1436-4466
Identifier
NCT03922750
Link
https://clinicaltrials.gov/study/NCT03922750
Phase
Phase II
Status
Completed
Sponsor
Novo Nordisk A/S
More details
This study compares insulin 287 (a possible new medicine) to insulin glargine (a medicine doctors can already prescribe) in people with type 2 diabetes. Different ways of switching from the insulin which the participants are already on to insulin 287 are also compared. This is done to find the best way to switch to insulin 287. The participants will either get insulin 287 that they will have to inject once a week or insulin glargine that they will have to inject once a day. Which treatment any participant gets is decided by chance. The study will last for about 5 months (23 weeks). The participants will have 14 clinic visits and 6 phone calls with the study doctor. At 3 of the clinic visits participants will be asked not to eat or drink anything (except for water) in the last 8 hours befor
Purpose
A Research Study in People With Type 2 Diabetes to Compare Two Types of Insulin: Insulin 287 and Insulin Glargine
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-05-09
Anticipated Date of Last Follow-up
2022-01-06
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2019-12-19
Actual Completion Date
2020-01-27
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion criteria: * Male or female, aged 18-75 years (both inclusive) at the time of signing informed consent. * Diagnosed with type 2 diabetes mellitus greater than or equal to 180 days prior to the day of screening. * Glycosylated haemoglobin (HbA1c) of 7.0-10.0% (53.0-85.8 mmol/mol) (both inclusive) as assessed by central laboratory. * Treated with once daily or twice daily basal insulin analogue (insulin degludec, insulin detemir, insulin glargine U100 or U300, total daily dose of 10-50 U, both inclusive) greater than or equal to 90 days prior to the day of screening. * Stable daily dose(s) for 90 days prior to the day of screening of any of the following antidiabetic drug(s) or combination regime(s): 1. Any metformin formulations greater than or equal to 1500 mg or maximum toler
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
154
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
NN1436-4383
Identifier
NCT03751657
Link
https://clinicaltrials.gov/study/NCT03751657
Phase
Phase II
Status
Completed
Sponsor
Novo Nordisk A/S
More details
The study compares 2 medicines for people with type 2 diabetes: insulin 287 (a new medicine) and insulin glargine (a medicine doctors can already prescribe). The study doctors will test insulin 287 to see how well it works compared to insulin glargine. The study will also test if insulin 287 is safe. The study participants will either get insulin 287 or insulin glargine (100 units/mL) - which treatment the participants get is decided by chance. The participants will need to inject their selves every day about the same time. Once a week the participant will need to take 1 extra injection on the same day of the week. The participants will have 16 clinic visits and 14 phone calls with the study doctor. During the study, the doctors will ask you to: 1) measure your blood sugar every day with a
Purpose
A Research Study to Compare Insulin 287 Once a Week to Insulin Glargine (100 Units/mL) Once a Day in People With Type 2 Diabetes.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2018-11-29
Anticipated Date of Last Follow-up
2021-03-05
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2019-12-16
Actual Completion Date
2020-01-17
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female, aged 18-75 years (both inclusive) at the time of signing informed consent * Diagnosed with type 2 diabetes mellitus greater than or equal to 180 days prior to the day of screening * HbA1c of 7.0-9.5% (53-80 mmol/mol) (both inclusive) as assessed by central laboratory * Stable daily dose(s) for 90 days prior to the day of screening of any of the following antidiabetic drug(s) or combination regime(s): Any metformin formulations greater than or equal to 1500 mg or maximum tolerated or effective dose (as documented in subject's medical record) OR Any metformin formulations greater than or equal to 1500 mg or maximum tolerated or effective dose (as documented in subject medical record) with Dipeptidyl peptidase-4 inhibitor (DPP4i) (greater than or equal t
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
247
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
ONWARDS 9
Identifier
NCT05823948
Link
https://clinicaltrials.gov/study/NCT05823948
Phase
Phase III
Status
Completed
Sponsor
Novo Nordisk A/S
More details
This study looks at how a person with type 2 diabetes can be treated with insulin icodec and a flash glucose monitor (a small sensor inserted under the skin to measure blood sugar all the time). The study will look at how well insulin icodec controls blood sugar when used in combination with a flash glucose monitor. Participants will get insulin icodec that they have to inject once a week on the same day of the week. The insulin will be injected with a needle in a skin fold in the thigh, upper arm, or stomach. The study will last for about 8 months. Participants will have to wear a flash glucose monitor throughout the study. This is a sensor that fits on arm. Participants will be asked to use a commercially available app called LibreView to allow team to view flash glucose monitor data. Pa
Purpose
A Study Using Flash Glucose Measurements for a New Once-weekly Insulin (Insulin Icodec) in People With Type 2 Diabetes Who Have Not Used Insulin Before (ONWARDS 9)
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-04-11
Anticipated Date of Last Follow-up
2025-03-05
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2024-03-06
Actual Completion Date
2024-04-11
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Informed consent obtained before any study-related activities. Study-related activities are any procedures that are carried out as part of the study, including activities to determine suitability for the study * Age above or equal to 18 years at the time of signing informed consent * Diagnosed with type 2 diabetes (T2D) greater than or equal to (\>=) 180 days before screening * HbA1c from 7.0%-11.0% (53.0-96.7 millimoles per mole \[mmol/mol\]) both inclusive at screening confirmed by central laboratory analysis * Insulin-naïve. However, short term insulin treatment for a maximum of 14 consecutive days before screening is allowed, as is prior insulin treatment for gestational diabetes * Stable daily dose(s) \>=90 days before screening of any of the following antidiabe
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
51
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
NN1436-7724
Identifier
NCT06340854
Link
https://clinicaltrials.gov/study/NCT06340854
Phase
Phase III
Status
Not provided
Sponsor
Novo Nordisk A/S
More details
This study compares insulin icodec, a new insulin taken once a week, to insulin glargine, an insulin taken once a day. The study medicine will be investigated in participants with type 2 diabetes. Participants will either get insulin icodec or insulin glargine. Which treatment participants get is decided by chance. Insulin icodec is the new medicine being tested, while insulin glargine is already approved and can be prescribed by doctors. Participants will get one injection of insulin icodec once a week, or one injection of insulin glargine once a day, depending on the treatment group participants are assigned into. Participants will use a pen with a small needle to inject the medicine under participants skin into participants thigh, upper arm or stomach.The study will last for about 9 mon
Purpose
A Research Study to See How Switching From a Daily Basal Insulin to a New Weekly Insulin, Insulin Icodec, Helps in Reducing the Blood Sugar Compared to Daily Insulin Glargine in Adults With Type 2 Dia
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2024-04-19
Anticipated Date of Last Follow-up
2025-03-13
Estimated Primary Completion Date
2025-05-16
Estimated Completion Date
2025-06-20
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Diagnosed with T2D greater than equal to (≥) 180 days prior to the day of screening. * HbA1c from 7.0-10.0% (53.0-85.8 mmol/mol), both inclusive, at screening confirmed by central laboratory analysis. * Treated with once-daily or twice-daily basal insulin (Neutral Protamine Hagedorn insulin, insulin degludec, insulin detemir, insulin glargine 100 U/mL, or insulin glargine 300 U/mL) ≥ 90 days prior to the day of screening with or without any of the following anti-diabetic drugs/regimens with stable doses greater than equal to (≥) 90 days prior to screening: metformin, sulfonylureas, meglitinides (glinides), Dipeptidyl peptidase 4 (DPP-4) inhibitors, Sodium-Glucose Transport Protein 2 (SGLT2) inhibitors, thiazolidinediones, alphaglucosidase inhibitors, oral combination
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
404
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
ONWARDS 2
Identifier
NCT04770532
Link
https://clinicaltrials.gov/study/NCT04770532
Phase
Phase III
Status
Completed
Sponsor
Novo Nordisk A/S
More details
This study compares insulin icodec (a new insulin taken once a week) to insulin degludec (an insulin taken once daily which is already available on the market) in people with type 2 diabetes. The study will look at how well insulin icodec taken weekly controls blood sugar compared to insulin degludec taken daily. Participants will either get insulin icodec that participants will have to inject once a week on the same day of the week or insulin degludec that participants will have to inject once a day at the same time every day. Which treatment participants get is decided by chance. The insulin is injected with a needle in a skin fold in the thigh, upper arm or stomach. The study will last for about 8 months. Participants will have 17 clinic visits and 13 phone calls with the study docto
Purpose
Compare Insulin Icodec and Insulin Degludec, in People With Type 2 Diabetes Who Use Daily Insulin
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-03-05
Anticipated Date of Last Follow-up
2025-01-28
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-01-27
Actual Completion Date
2022-03-01
Studied populations
Age Cohort
- Children
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female aged above or equal to 18 years at the time of signing informed consent. * Diagnosed with T2D greater than or equal to 180 days prior to the day of screening. * HbA1c from 7.0-10.0% (53.0 85.8 mmol/mol) both inclusive at screening confirmed by central laboratory analysis. * Treated with once daily or twice daily basal insulin (Neutral Protamine Hagedorn insulin, insulin degludec, insulin detemir, insulin glargine 100 units/mL, or insulin glargine 300 units/mL): greater than or equal to 90 days prior to the day of screening with or without any of the following anti-diabetic drugs/regimens with stable doses greater than or equal to 90 days prior to screening: * Metformin * Sulfonylureas * Meglitinides (glinides) * DPP-4 inhibitors * SGLT2 inhi
Health status
Study type
Interventional (clinical trial)
Enrollment
526
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
ONWARDS 3
Identifier
NCT04795531
Link
https://clinicaltrials.gov/study/NCT04795531
Phase
Phase III
Status
Completed
Sponsor
Novo Nordisk A/S
More details
This study compares insulin icodec (a new insulin taken once a week) to insulin degludec (an insulin taken once daily which is already available on the market) in people with type 2 diabetes. The study will look at how well insulin icodec taken weekly controls blood sugar compared to insulin degludec taken daily. Participants will get their study medicine in an injection pen. Participants will get a pen for weekly injection and one for daily injection. One will be icodec or degludec and the other will be dummy medicine. The treatment participants get is decided by chance. Participants and the study staff will not know which active medicine they get. The insulin is injected with a needle in a skin fold in the thigh. The study could last for about 8 months. Participants will have 13 clini
Purpose
Compare Insulin Icodec and Insulin Degludec, in People With Type 2 Diabetes Who Have Not Used Insulin Before
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-03-24
Anticipated Date of Last Follow-up
2024-11-10
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-06-23
Actual Completion Date
2022-06-23
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female aged above or equal to 18 years at the time of signing informed consent. * Diagnosed with T2D (type 2 diabetes) greater than or equal to 180 days prior to the day of screening. * HbA1c (glycated haemoglobin) from 7.0-11.0% (53.0-96.7 mmol/mol) both inclusive at screening confirmed by central laboratory analysis. * Insulin naïve. However, short term insulin treatment for a maximum of 14 days prior to the day of screening is allowed, as is prior insulin treatment for gestational diabetes. * Stable daily dose(s) greater than or equal to 90 days prior to the day of screening of any of the following anti-diabetic drug(s) or combination regimen(s): a.) Any metformin formulations greater than or equal to 1500 mg or maximum tolerated or effective dose. b.)
Health status
Study type
Interventional (clinical trial)
Enrollment
588
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
COMBINE 1
Identifier
NCT05352815
Link
https://clinicaltrials.gov/study/NCT05352815
Phase
Phase III
Status
Completed
Sponsor
Novo Nordisk A/S
More details
This study will compare the new medicine IcoSema, which is a combination of insulin icodec and semaglutide, taken once a week, to insulin icodec taken once a week in people with type 2 diabetes. The study will look at how well IcoSema controls blood sugar level in people with type 2 diabetes compared to insulin icodec. Participants will either get IcoSema or insulin icodec. Which treatment participants get is decided by chance. IcoSema and insulin icodec are both new medicines that doctors cannot prescribe. Participants will get IcoSema or insulin icodec, which participants must inject once a week with a pen, which has a small needle, in a skin fold in the thigh, upper arm, or stomach. The study will last for about 1 year and 1 month. Participants will have 21 clinic visits, 31 phone/v
Purpose
A Research Study to See How Well Weekly Medicine IcoSema,, Controls Blood Sugar Level in People With Type 2 Diabetes Compared to Weekly Insulin Icodec
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-06-01
Anticipated Date of Last Follow-up
2025-03-18
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2024-03-19
Actual Completion Date
2024-04-23
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Key inclusion criteria 1. Male or female and age above or equal to 18 years at the time of signing informed consent. 2. Diagnosed with type 2 diabetes mellitus 180 days or more before screening. 3. HbA1c of 7.0 10.0% (53.0 85.8 mmol/mol) (both inclusive) as assessed by central laboratory on the day of screening. 4. Treated with once daily or twice daily basal insulin (neutral protamine hagedorn insulin, insulin degludec, insulin detemir, insulin glargine 100 units/mL, or insulin glargine 300 units/mL) 20- 80 units/day for 90 days or more before screening. Short term bolus insulin treatment for a maximum of 14 days before screening is allowed, as is prior insulin treatment for gestational diabetes. The treatment can be with or without any of the following anti diabetic drugs with stable do
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
1291
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
ONWARDS 6
Identifier
NCT04848480
Link
https://clinicaltrials.gov/study/NCT04848480
Phase
Phase III
Status
Completed
Sponsor
Novo Nordisk A/S
More details
This study compares insulin icodec (a new insulin) to insulin degludec (an insulin already available on the market) in people with type 1 diabetes. The study will look at how well insulin icodec taken weekly controls blood sugar compared to insulin degludec taken daily. Participants will either get insulin icodec that participants will have to inject once a week on the same day of the week, or insulin degludec that participants will have to inject once a day at the same time every day. Which treatment participants get is decided at random. Participants will also get a mealtime insulin. The insulin is injected with a needle in a skin fold in the thigh, upper arm or stomach. The study will last for about 1 year and 2 months. Participants will have 28 clinic visits and 28 phone calls with
Purpose
A Research Study to Compare a New Weekly Insulin, Insulin Icodec, and an Available Daily Insulin, Insulin Degludec, Both in Combination With Mealtime Insulin in People With Type 1 Diabetes (ONWARDS 6)
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-04-30
Anticipated Date of Last Follow-up
2024-09-06
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-04-28
Actual Completion Date
2022-12-02
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female aged greater than or equal to 18 years at the time of signing informed consent. * Diagnosed with type 1 diabetes mellitus greater than or equal to 1 year prior to the day of screening. * Treated with multiple daily insulin injections (basal and bolus insulin analogue regimes) greater than or equal to 1 year prior to the day of screening. * HbA1c below10% at screening visit based on analysis from central laboratory. Exclusion Criteria: * Myocardial infarction, stroke, hospitalization for unstable angina pectoris or transient ischaemic attack within 180 days prior to the day of screening. * Chronic heart failure classified as New York Heart Association (NYHA) Class IV at screening. * Anticipated initiation or change in concomitant medications (for more
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
582
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
ONWARDS 4
Identifier
NCT04880850
Link
https://clinicaltrials.gov/study/NCT04880850
Phase
Phase III
Status
Completed
Sponsor
Novo Nordisk A/S
More details
This study compares insulin icodec (a new insulin taken once a week) to insulin glargine (an insulin taken once daily which is already available on the market) in people with type 2 diabetes. The study will look at how well insulin icodec taken weekly controls blood sugar compared to insulin glargine taken daily. Participants will either get insulin icodec that participants will have to inject once a week on the same day of the week or insulin glargine that participants will have to inject once a day at the same time every day. Which treatment participants will get is decided by chance. Participants will also get a mealtime insulin.The insulin is injected with a needle in a skin fold in the thigh, upper arm or stomach. The study will last for about 8 months. participants will have 17 cl
Purpose
Compare Insulin Icodec and Insulin Glargine, Both in Combination With Mealtime Insulin, in People With Type 2DM previously on basal-bolus therapy
Interventions
Not provided
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-05-14
Anticipated Date of Last Follow-up
2024-05-27
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-06-16
Actual Completion Date
2022-06-16
Studied populations
Age Cohort
- Children
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female aged above or equal to 18 years at the time of signing informed consent. * Diagnosed with type 2 diabetes mellitus (T2D) greater than or equal to 180 days prior to the day of screening. * Glycated haemoglobin (HbA1c) from 7.0-10.0% (53.0 85.8 mmol/mol) both inclusive at screening confirmed by central laboratory analysis. * Treated with once daily basal insulin (neutral protamine hagedorn insulin, insulin degludec, insulin detemir, insulin glargine 100 units/mL, or insulin glargine 300 units/mL) and 2-4 daily injections of bolus insulin analog (insulin aspart, faster acting insulin aspart, insulin lispro, insulin glulisine) greater than or equal to 90 days prior to the day of screening with or without any of the following anti-diabetic drugs/regimens wi
Health status
Study type
Interventional (clinical trial)
Enrollment
582
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
ONWARDS 5
Identifier
NCT04760626
Link
https://clinicaltrials.gov/study/NCT04760626
Phase
Phase III
Status
Completed
Sponsor
Novo Nordisk A/S
More details
This study compares insulin icodec to different daily insulins in people with type 2 diabetes. The study will look at how well insulin icodec taken once weekly controls blood sugar compared to the insulins taken once daily. Participants will either get insulin icodec, that participants will have to inject once a week on the same day of the week, or a marketed insulin, that participants will have to inject once a day. Which treatment participants get is decided at random. The insulin is injected with a needle in a skin fold in the thigh, upper arm or stomach. Participants will measure their blood sugar every day. Participants will get a study phone to record safety data in the electronic diary (eDiary). If participants get a daily insulin they will record their insulin doses in the eDiar
Purpose
Compare Insulin Icodec Used With DoseGuide App, and Daily Insulins in People With Type 2 Diabetes Who Have Not Used Insulin Before
Interventions
Not provided
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-03-01
Anticipated Date of Last Follow-up
2024-08-06
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-08-12
Actual Completion Date
2022-08-29
Studied populations
Age Cohort
- Children
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Informed consent obtained before any trial-related activities. Trial-related activities are any procedures that are carried out as part of the trial, including activities to determine suitability for the trial. * Male or female. * Age above or equal to 18 years at the time of signing informed consent. * Diagnosed with T2D greater than or equal to 180 days prior to the day of screening. * HbA1c above 7.0% (53 mmol/mol) as measured by central lab. * Insulin naïve. However, short term insulin treatment for a maximum of 14 days prior to the day of screening is allowed, as is prior insulin treatment for gestational diabetes. * Stable daily dose(s) greater than or equal to 90 days prior to the day of screening of any of the following antidiabetic drug(s) or combination reg
Health status
Study type
Interventional (clinical trial)
Enrollment
1085
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
COMBINE 4
Identifier
NCT06269107
Link
https://clinicaltrials.gov/study/NCT06269107
Phase
Phase III
Status
Not provided
Sponsor
Novo Nordisk A/S
More details
This study will compare the new medicine IcoSema, which is a combination of insulin icodec and semaglutide, taken once a week, to insulin glargine (mentioned as insulin glargine in this form) taken daily in people with type 2 diabetes. The study will look at how well IcoSema controls blood sugar levels as compared to insulin glargine in people with type 2 diabetes who do not have their blood sugar properly controlled with other oral diabetes medicines. Participant will either get IcoSema or insulin glargine. Which treatment participants get is decided by chance. IcoSema is a new medicine that doctors cannot prescribe. Doctors can already prescribe insulin glargine in many countries. The study will last for about 11 months (47 weeks).
Purpose
A Research Study to See How Well New Weekly Medicine IcoSema, Which is a Combination of Insulin Icodec and Semaglutide, Controls Blood Sugar Levels in People With Type 2 Diabetes (T2D), Compared to Da
Interventions
Not provided
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2024-02-15
Anticipated Date of Last Follow-up
2025-01-13
Estimated Primary Completion Date
2025-05-26
Estimated Completion Date
2025-06-30
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female and age above or equal to 18 years at the time of signing the informed consent. * Diagnosed with T2D greater than or equal to (≥) 180 days before screening. * HbA1c ≥ 8.0% (≥ 64.0 millimoles per mole \[mmol/mol\]) as assessed by central laboratory on the day of screening. * Insulin naïve. Short term insulin treatment for a maximum of 14 consecutive days before screening is allowed, as is prior insulin treatment for gestational diabetes. * Currently treated with 1-3 oral anti diabetic drug (OADs) with stable daily doses ≥ 90 days before screening comprising any of the following anti diabetic drug(s) at effective or maximum tolerated dose. * Metformin * Sulfonylureas * Meglitinides (glinides) * Dipeptidyl peptidase (DPP) 4 inhibitors * Sodium
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
474
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
Excipients
Proprietary excipients used
Not provided
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
Not provided
Residual solvents used
Not provided
Patent info
Description
Icodec formulation
Brief description
The invention provides a pharmaceutical composition comprising insulin icodec in a unique combination of excipients carefully formulated in order to reduce formation of oligomers at the injection site, while still showing PK/PD properties suited for a once-weekly administration. In another aspect, the invention provides a pharmaceutical composition with decreased viscosity upon injection and accordingly decreased propensity to create any discomfort upon injection. In another aspect, the invention provides a pharmaceutical composition with improved stability. In another aspect, the invention provides a pharmaceutical composition for use as a medicament for the treatment of a metabolic disorder.
Representative patent
WO2018109162
Category
Composition
Patent holder
Novo Nordisk AS
Exclusivity
Not provided
Expiration date
December 15, 2037
Status
Granted in: CN, RU, ID, EP, VN, ZA, US; Filed in: BR, IN, PH, TH
Description
Icodec formulation
Brief description
A stable pharmaceutical formulation containing an insulin icodec that can conveniently be prepared by adding glycerol, phenol, m-cresol and zinc ions to it. Another aspect of this invention relates to the furnishing of insulin formulations having a relatively high content of insulin, e.g., a concentration of insulin above about 1 .5 mM insulin, preferably above about 3 mM insulin, and more preferred above about 4 mM and a concentration below about 9 mM insulin. Another aspect of this invention relates to the furnishing of insulin formulations having a sufficient chemical stability, a sufficient physical stability, a sufficiently low viscosity, a sufficient solubility and a sufficient stable oligomerisation pattern.
Representative patent
WO2013153000
Category
Composition
Patent holder
Novo Nordisk AS
Exclusivity
Not provided
Expiration date
April 5, 2033
Status
Granted in CN, US, EP Filed in: RU Not in force: BR
Description
Insulin Icodec compound and analogues
Brief description
Novel acylated insulin analogues exhibiting resistance towards proteases can, effectively, be administered pulmonary or orally. The insulin analogues contain B25H and A14E or A14H.
Representative patent
WO2009115469
Category
Compound
Patent holder
Novo Nordisk AS
Exclusivity
Not provided
Expiration date
March 13, 2029
Status
Granted: AU, BR, CN, IN, JP, MX, RU, ZA, EP, KR, US
Supporting material
Publications
Kjeldsen, T. B., Hubálek, F., Hjørringgaard, C. U., Tagmose, T. M., Nishimura, E., Stidsen, C. E., Porsgaard, T., Fledelius, C., Refsgaard, H. H. F., Gram-Nielsen, S., Naver, H., Pridal, L., Hoeg-Jensen, T., Jeppesen, C. B., Manfè, V., Ludvigsen, S., Lautrup-Larsen, I., & Madsen, P. (2021). Molecular Engineering of Insulin Icodec, the First Acylated Insulin Analog for Once-Weekly Administration in Humans. Journal of medicinal chemistry, 64(13), 8942–8950. https://doi.org/10.1021/acs.jmedchem.1c00257
Here, we describe the molecular engineering of insulin icodec to achieve a plasma half-life of 196 h in humans, suitable for once-weekly subcutaneously administration. Insulin icodec is based on re-engineering of the ultra-long oral basal insulin OI338 with a plasma half-life of 70 h in humans. This systematic re-engineering was accomplished by (1) further increasing the albumin binding by changing the fatty diacid from a 1,18-octadecanedioic acid (C18) to a 1,20-icosanedioic acid (C20) and (2) further reducing the insulin receptor affinity by the B16Tyr → His substitution. Insulin icodec was selected by screening for long intravenous plasma half-life in dogs while ensuring glucose-lowering potency following subcutaneous administration in rats. The ensuing structure-activity relationship resulted in insulin icodec. In phase-2 clinical trial, once-weekly insulin icodec provided safe and efficacious glycemic control comparable to once-daily insulin glargine in type 2 diabetes patients. The structure-activity relationship study leading to insulin icodec is presented here.
Hubálek, F., Cramer, C. N., Helleberg, H., Johansson, E., Nishimura, E., Schluckebier, G., Steensgaard, D. B., Sturis, J., & Kjeldsen, T. B. (2024). Enhanced disulphide bond stability contributes to the once-weekly profile of insulin icodec. Nature communications, 15(1), 6124. https://doi.org/10.1038/s41467-024-50477-9
Insulin icodec is a once-weekly insulin analogue that has a long half-life of approximately 7 days, making it suitable for once weekly dosing. The Insulin icodec molecule was developed based on the hypothesis that lowering insulin receptor affinity and introducing a strong albumin-binding moiety would result in a long insulin half-life, provided that non-receptor-mediated clearance is diminished. Here, we report an insulin clearance mechanism, resulting in the splitting of insulin molecules into its A-chain and B-chain by a thiol-disulphide exchange reaction. Even though the substitutions in insulin icodec significantly stabilise insulin against such degradation, some free B-chain is observed in plasma samples from minipigs and people with type 2 diabetes. In summary, we identify thiol-disulphide exchange reactions to be an important insulin clearance mechanism and find that stabilising insulin icodec towards this reaction significantly contributes to its long pharmacokinetic/pharmacodynamic profile.
Additional documents
Useful links
There are no additional links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Not provided