
|
Developed by 

|
Supported by 

|
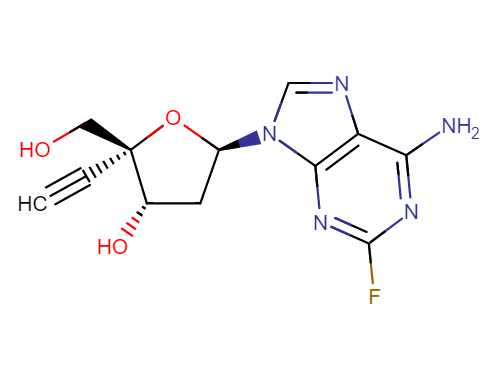
Islatravir (ISL)
Developer(s)

|
Merck & Co., Inc. Originator
https://www.msd.com/
United States Merck & Co., Inc. is an American multinational pharmaceutical company known as Merck Sharp & Drone (MSD) in territories outside of the USA and Canada. Merck was originally established in 1891, and is headquartered in Rahway, New Jersey. The company is particularly well known for developing and manufacturing biologic therapies, vaccines, medicines and animal health products. |
Drug structure

Islatravir Chemical Structure
Sourced from DrugBank
Drug information
Associated long-acting platforms
Polymeric implant, Oral solid form
Administration route
Oral, Subcutaneous
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
Frequency of administration
Not provided
User acceptance
Not provided
Dosage
Available dose and strength
investigational
Maximum dose
Not provided
Recommended dosing regimen
Not provided
Additional comments
Not provided
Dosage link(s)
Not provided
Drug information
Drug's link(s)
Generic name
Brand name
Compound type
Drug class/category
Summary
Approval status
Regulatory authorities
Delivery device(s)
Not provided
Scale-up and manufacturing prospects
Scale-up prospects
The automated lab reactor platforms EasyMax 102 and 402 (Mettler-Toledo AG, AutoChem, Switzerland) were utilised by Merck for the reaction scale-up of Islatravir synthesis. The EasyMax reactors contained integrated measurement probes for pH and temperature, FireStringO2 dissolved oxygen sensors (Pyro-Science GmbH, Germany) and the EasySampler 1210 automated sampling system.
Tentative equipment list for manufacturing
EasyMax 102 and 402 equipped with FireStringO2 sensors and the EasySampler 1210 system. A thermal gas flow controller (Aalborg, USA) to monitor and control oxidation air-gas flow to the reactor, with a suitable compressed air-source. Reactions at the 1L scale should be conducted using the Optimal 1001 automated lab reactor platform (Mettler-Toledo) or equivalent, alongside a sintered steel frit for improved 1L gas distribution for the oxidation reactions.
Manufacturing
Several synthetic chemical processes describing the manufacture of ISL have been published, with each approach containing around twelve and eighteen steps. However, it should be noted that these approaches have proved to be complex and highly inefficient, with marked difficulty in controlling 2’-deoxyribonucleoside anomer stereochemistry and the requirement for several protecting-group manipulations. To counter these issues, Merck developed a highly innovative and extraordinarily efficient approach utilising directed evolution to create a novel three-step biocatalytic cascade for ISL synthesis
Specific analytical instrument required for characterization of formulation
400 MHz Briker AVANCE III and 500MHz Bruker Ultrashield spectrometer (or equivalent) for 1H, 19F, 31P and 13C NMR. An Accurate-Mass Time-of-Flight (TOF) high resolution mass spectrometer. Molecular Devices plate reader Spectra Max Plus for Spectrophotomeric analyses, alongside a Perkin Elmer polarimeter with a PCB 1500 water Peltier system for optical rotation measurements. Aglient 7890A instrument for gas chromatography. UPLC via Agilent Technologies 1290 Infinity II series or HPLC through Agilent 1100 Series. Corona Ultra RS detector by Dionex for supercritical fluid chromatography.
Clinical trials
MK-8591-016
Identifier
NCT04003103
Link
https://clinicaltrials.gov/study/NCT04003103
Phase
Phase II
Status
Completed
Sponsor
Merck Sharp & Dohme LLC
More details
Not provided
Purpose
This study will evaluate the safety, tolerability and pharmacokinetics (PK) of 6 once-monthly doses of oral islatravir (60 mg and 120 mg) compared with placebo in adults at low risk of HIV-1 infection
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-09-19
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-03-18
Actual Completion Date
2022-11-24
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: - Is in general good health with acceptable laboratory values at screening. - Is confirmed HIV-uninfected based on negative HIV-1/HIV-2 test result before randomization. - Has low risk of HIV infection, within 12 months prior to screening visit or the rescreening visit (if applicable). - Use contraceptives consistent with local regulations. - Female is not pregnant or breastfeeding, and is not a woman of childbearing potential (WOCBP). - A WOCBP is using an acceptable contraceptive method, or is abstinent from heterosexual intercourse as their preferred and usual lifestyle; or has a negative pregnancy test.
Health status
Study type
Interventional (clinical trial)
Enrollment
242
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Double-blind masking
Masking description
Double (Participant, Investigator)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key resources
| Type | Title | Content | Link |
|---|---|---|---|
| Link | Safety and pharmacokinetics of oral islatravir once monthly for HIV pre-exposure prophylaxis (PrEP): week 24 analysis of a phase 2a trial | https://theprogramme.ias2021.org/Abstract/Abstract/2361 |
IMPOWER-022
Identifier
NCT04644029
Link
https://clinicaltrials.gov/study/NCT04644029
Phase
Phase III
Status
Terminated
Sponsor
Merck Sharp & Dohme LLC
More details
Voluntarily terminated due to benefit/risk assessment.
Purpose
This study will evaluate whether oral islatravir (ISL) is effective in preventing Human Immunodeficiency Virus Type 1 (HIV-1) infection in women at high-risk for HIV-1 infection.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-02-24
Anticipated Date of Last Follow-up
2024-08-06
Estimated Primary Completion Date
Not provided
Estimated Completion Date
2024-07-01
Actual Primary Completion Date
2023-07-18
Actual Completion Date
2024-06-11
Studied populations
Age Cohort
- Adolescents
- Adults
Genders
- Cisgender female
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: - Confirmed HIV-uninfected based on negative HIV-1/HIV-2 test results before randomization. - Sexually active (vaginal and/or anal sex) with a male sexual partner in the 30 days prior to screening. - High risk for HIV-1 infection. - Not pregnant or breastfeeding, and one of the following conditions applies: - Not a woman of childbearing potential (WOCBP) or is a WOCBP and is using an acceptable contraceptive method during the intervention period and for at least 42 days after the last dose. - A WOCBP must have a negative pregnancy test within 24 hours prior to the first dose of study intervention.
Health status
Study type
Interventional (clinical trial)
Enrollment
730
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Triple (Participant, Care Provider, Investigator)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key resources
IMPOWER-024
Identifier
NCT04652700
Link
https://clinicaltrials.gov/study/NCT04652700
Phase
Phase III
Status
Completed
Sponsor
Merck Sharp & Dohme LLC
More details
Voluntarily terminated due to benefit/risk assessment.
Purpose
Evaluate the safety and tolerability of oral Islatravir (ISL) once monthly as Preexposure Prophylaxis.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-03-15
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2023-08-04
Actual Completion Date
2023-08-04
Studied populations
Age Cohort
- Adolescents
- Adults
- Older Adults
Genders
- Cisgender male
- Transgender female
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Cisgender men who have sex with men (MSM) and transgender women (TGW) who have sex with men.
Health status
Study type
Interventional (clinical trial)
Enrollment
494
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Double-blind masking
Masking description
In Study Part 1, double-blind with in-house blinding is used. In Study Part 2, sponsor personnel not directly involved with blinded safety monitoring will be unblinded to participants' randomized study intervention in Part 1 (personnel involved with Part 2 will remain blinded). In Study Part 3, al participants, investigators, and Sponsor personnel are unblinded as to the participant's original randomized intervention group.
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key resources
MK-8591-035
Identifier
NCT05130086
Link
https://clinicaltrials.gov/study/NCT05130086
Phase
Phase II
Status
Withdrawn
Sponsor
Merck Sharp & Dohme LLC
More details
Withdrawn due to Business Reasons.
Purpose
Evaluate the safety and tolerability of Islatravir (ISL) in trans and gender diverse participants who are receiving gender-affirming hormone therapy and are at low-risk for HIV-1 infection.
Interventions
Intervention 1
Countries
Not provided
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2022-10-17
Actual Start Date
Not provided
Anticipated Date of Last Follow-up
2022-10-13
Estimated Primary Completion Date
2024-03-25
Estimated Completion Date
2024-03-25
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Transgender female
- Transgender male
- Intersex
- Gender non-binary
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Study participants must identify with a gender that is different from that assigned at birth.
Health status
Study type
Interventional (clinical trial)
Enrollment
Not provided
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key resources
MK-8591-043
Identifier
NCT05115838
Link
https://clinicaltrials.gov/study/NCT05115838
Phase
Phase II
Status
Withdrawn
Sponsor
Merck Sharp & Dohme LLC
More details
Withdrawn for business reasons.
Purpose
Evaluate the safety, tolerability, and pharmacokinetics (PK) of an islatravir (ISL)-eluting implant
Interventions
Intervention 1
Intervention 2
Countries
Not provided
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2024-01-04
Actual Start Date
Not provided
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
2025-10-02
Estimated Completion Date
2025-10-02
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: - Is in good health. - Is confirmed human immunodeficiency virus (HIV)-uninfected. - Is at low risk of HIV infection. - For males, uses contraception in accordance with local regulations regarding contraception use for those participating in clinical trials. - For females, is not pregnant or breastfeeding and one of the following applies: (i) Is not a participant of childbearing potential (POCBP). (ii) Is a POCBP and uses an acceptable contraception method or is abstinent.
Health status
Study type
Interventional (clinical trial)
Enrollment
Not provided
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Triple (Participant, Investigator, Outcomes Assessor)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key resources
MK-8591-003
Identifier
NCT02217904
Link
https://clinicaltrials.gov/study/NCT02217904
Phase
Phase I
Status
Completed
Sponsor
Merck Sharp & Dohme LLC
More details
Not provided
Purpose
Evaluate the safety, tolerability, pharmacokinetics, and anti-retroviral therapy activity of Islatravir (MK-8591) monotherapy in ART-naive, HIV-1 infected participants.
Interventions
Intervention 1
Countries
Not provided
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2015-09-17
Anticipated Date of Last Follow-up
2019-07-24
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2017-05-11
Actual Completion Date
2017-05-11
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: - Non-pregnant, non-breast feeding, postmenopausal or surgically sterile female. - Female with reproductive potential agrees to use (or have male partner use) two acceptable methods of birth control. - Male agrees to use acceptable method of contraception during study and for 90 days after last dose of trial drug. - Has stable baseline health, other than HIV infection. - Has no significantly abnormal electrocardiogram. - Is HIV-1 positive. - Have a screening plasma HIV-1 RNA ≥ 10,000 copies/mL within 30 days prior to the treatment phase of this study. For inclusion in Panel Islatravir Extended Observation, participants must also have a screening plasma HIV-1 RNA ≤ 25,000 copies/mL within 30 days prior to the treatment phase. - Is ART naive.
Health status
Study type
Interventional (clinical trial)
Enrollment
30
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
| Type | Title | Content | Link |
|---|---|---|---|
| Link | Safety, pharmacokinetics, and antiretroviral activity of islatravir (ISL, MK-8591), a novel nucleoside reverse transcriptase translocation inhibitor, following single-dose administration | https://doi.org/10.1016/s2352-3018(19)30372-8 |
MK-8591-007
Identifier
EudraCT: 2018-001329-18
Link
https://www.nature.com/articles/s41591-021-01479-3
Phase
Phase I
Status
Completed
Sponsor
Merck Sharp & Dohme LLC
More details
Not provided
Purpose
Safety and pharmacokinetics of islatravir subdermal implant for HIV-1 pre-exposure prophylaxis: a randomized, placebo-controlled phase 1 trial
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2018-06-04
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2018-07-05
Actual Completion Date
2019-01-25
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Not provided
Health status
Study type
Interventional (clinical trial)
Enrollment
16
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Double-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key resources
| Type | Title | Content | Link |
|---|---|---|---|
| Link | Safety and pharmacokinetics of islatravir subdermal implant for HIV-1 pre-exposure prophylaxis: a randomized, placebo-controlled phase 1 trial | https://www.nature.com/articles/s41591-021-01479-3 |
Excipients
Proprietary excipients used
Not provided
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
Not provided
Residual solvents used
Not provided
Patent info
Compound patent families
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Islatravir for the treatment or prophylaxis of HIV (dosing regimen less frequent than once-daily)
Expiry date: 2037-02-10 The present invention is directed to methods for inhibition of HIV reverse transcriptase, treatment of infection by HIV, prophylaxis of infection by HIV, and the treatment, prophylaxis and/or delay in the onset or progression of AIDS or ARC by administering a compound of structural Formula (I) or a pharmaceutically acceptable salt or co-crystal thereof, wherein X is -F or -Cl, less frequently than once daily. |
WO2017139519 | Use | MERCK SHARP & DOHME [US] | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro, Serbia, Moldova, Republic of, Morocco, Dominican Republic, Belarus, Kazakhstan, Azerbaijan, Armenia, Mexico, South Africa, Georgia, Iran (Islamic Republic of), Ukraine, Malaysia, Botswana, Ghana, Kenya, Namibia, Tunisia, Jordan | Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, Croatia, Romania, Latvia, Lithuania, Slovenia, Australia, Canada, Russian Federation, Japan, Korea, Republic of, Taiwan, Province of China, United States of America, New Zealand |
| Filed | China, El Salvador, Nicaragua | Lithuania, Korea, Republic of, Singapore, Taiwan, Province of China, United States of America, Hong Kong, Israel |
| Not in force | Argentina, China, Turkmenistan, Tajikistan, Kyrgyzstan, Philippines, World Intellectual Property Organization (WIPO), Gambia (the), Lesotho, Malawi, Mozambique, Sierra Leone, Liberia, Rwanda, Sao Tome and Principe, Sudan, Eswatini, Tanzania, United Republic of, Uganda, Zambia, Zimbabwe, Brazil | Monaco, San Marino, Chile, Japan, Korea, Republic of, World Intellectual Property Organization (WIPO) |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Islatravir compound and use for treating HIV
Expiry date: 2025-03-24 A compound which has excellent anti-HIV activity, is effective also against polypharmacy-resistant HIV strains having resistance to two or more anti-HIV drugs, especially AZT, DDI, DDC, D4T, 3TC, etc., is lowly cytotoxic, and has resistance to deactivation by adenosine deaminase. It is a 4'-C-substituted 2-haloadenosine derivative represented by the following formula [I], [II], or [III]. Also provided is a medicinal composition comprising the derivative and a pharmaceutically acceptable support. [Chemical formula 1] (In the formulae, X represents halogeno; R<1> represents ethynyl or cyano; and R<2> represents hydrogen or the atoms of a residue of phosphoric acid or a derivative thereof.) |
WO2005090349 | Compound | Yamasa Corporation | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | United States of America | |
| Filed | ||
| Not in force | Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Serbia, World Intellectual Property Organization (WIPO), Mexico | Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Croatia, Romania, Latvia, Lithuania, Slovenia, Canada, Japan, United States of America, World Intellectual Property Organization (WIPO) |
Supporting material
Publications
Derbalah, Abdallaha,b; Karpick, Hayley Christineb,∗; Maize, Hollyb,∗; Skersick, Prestonb,∗; Cottrell, Mackenzieb; Rao, Gauri G.b. Role of islatravir in HIV treatment and prevention: an update. Current Opinion in HIV and AIDS: July 2022 - Volume 17 - Issue 4 - p 240-246
doi: https://doi.org/10.1097/COH.0000000000000740
Purpose of review
To summarize recent updates on the potential role of islatravir for HIV treatment and prevention.
Recent findings
Islatravir is an investigational antiretroviral agent with unique pharmacologic properties that facilitate flexible dosing regimens. Islatravir has demonstrated potent antiviral activity and a high barrier to resistance when combined with doravirine and lamivudine. A simplified two-drug HIV treatment regimen of islatravir combined with doravirine has also demonstrated comparable efficacy to standard of care three-drug regimens. The long half-life and high potency of islatravir's active metabolite may support its use as a long-acting option for HIV preexposure prophylaxis (PrEP). A once monthly oral dose of islatravir maintains effective concentrations of its active metabolite over the entire dosing interval. Furthermore, an investigational implantable formulation has been projected to provide efficacious concentrations for at least a year and exhibits comparable distribution into vaginal and rectal tissues making it a promising PrEP option for male and female individuals. Islatravir has minimal risks of drug interactions as it is not a substrate, inducer, or inhibitor of major drug metabolizers and transporters. Finally, clinical trials demonstrate islatravir's favorable safety profile revealing only mild and transient adverse events.
Summary
Leveraging the unique pharmacological properties of islatravir offers opportunities for simplified HIV treatment regimens and long-acting PrEP making it a valuable addition to the antiretroviral arsenal.
Beloor J, Kudalkar SN, Buzzelli G, Yang F, Mandl HK, Rajashekar JK, Spasov KA, Jorgensen WL, Saltzman WM, Anderson KS, Kumar P. Long-acting and extended-release implant and nanoformulations with a synergistic antiretroviral two-drug combination controls HIV-1 infection in a humanized mouse model. Bioeng Transl Med. 2021 Jun 26;7(1):e10237. DOI: https://doi.org/10.1002/btm2.10237. PMID: 35079625; PMCID: PMC8780078.
The HIV pandemic has affected over 38 million people worldwide with close to 26 million currently accessing antiretroviral therapy (ART). A major challenge in the long-term treatment of HIV-1 infection is nonadherence to ART. Long-acting antiretroviral (LA-ARV) formulations, that reduce dosing frequency to less than once a day, are an urgent need that could tackle the adherence issue. Here, we have developed two LA-ART interventions, one an injectable nanoformulation, and the other, a removable implant, for the delivery of a synergistic two-drug ARV combination comprising a pre-clinical nonnucleoside reverse transcriptase inhibitor (NNRTI), Compound I, and the nucleoside reverse transcriptase inhibitor (NRTI), 4′-ethynyl-2-fluoro-2′-deoxyadenosine. The nanoformulation is poly(lactide-co-glycolide)-based and the implant is a copolymer of ω-pentadecalactone and p-dioxanone, poly(PDL-co-DO), a novel class of biocompatible, biodegradable materials. Both the interventions, packaged independently with each ARV, released sustained levels of the drugs, maintaining plasma therapeutic indices for over a month, and suppressed viremia in HIV-1-infected humanized mice for up to 42 days with maintenance of CD4+ T cells. These data suggest promise in the use of these new drugs as LA-ART formulations in subdermal implant and injectable mode.
Huffman, M.A., Fryszkowska, A., Alvizo, O., Borra-Garske, M., Campos, K.R., Canada, K.A., Devine, P.N., Duan, D., Forstater, J.H., Grosser, S.T., Halsey, H.M., Hughes, G.J., Jo, J., Joyce, L.A., Kolev, J.N., Liang, J., Maloney, K.M., Mann, B.F., Marshall, N.M. and McLaughlin, M. (2019). Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science, 366(6470), pp.1255–1259. doi: https://doi.org/10.1126/science.aay8484.
Enzyme-catalyzed reactions have begun to transform pharmaceutical manufacturing, offering levels of selectivity and tunability that can dramatically improve chemical synthesis. Combining enzymatic reactions into multistep biocatalytic cascades brings additional benefits. Cascades avoid the waste generated by purification of intermediates. They also allow reactions to be linked together to overcome an unfavorable equilibrium or avoid the accumulation of unstable or inhibitory intermediates. We report an in vitro biocatalytic cascade synthesis of the investigational HIV treatment islatravir. Five enzymes were engineered through directed evolution to act on non-natural substrates. These were combined with four auxiliary enzymes to construct islatravir from simple building blocks in a three-step biocatalytic cascade. The overall synthesis requires fewer than half the number of steps of the previously reported routes.
McLaughlin M, Kong J, Belyk KM, Chen B, Gibson AW, Keen SP, Lieberman DR, Milczek EM, Moore JC, Murray D, Peng F, Qi J, Reamer RA, Song ZJ, Tan L, Wang L, Williams MJ. Enantioselective Synthesis of 4'-Ethynyl-2-fluoro-2'-deoxyadenosine (EFdA) via Enzymatic Desymmetrization. Org Lett. 2017 Feb 17;19(4):926-929. doi: https://doi.org/10.1021/acs.orglett.7b00091. Epub 2017 Feb 6. PMID: 28165251.
An enantioselective synthesis of the potent anti-HIV nucleoside EFdA is presented. Key features of stereocontrol include construction of the fully substituted 4'-carbon via a biocatalytic desymmetrization of 2-hydroxy-2-((triisopropylsilyl)ethynyl)propane-1,3-diyl diacetate and a Noyori-type asymmetric transfer hydrogenation to control the stereochemistry of the 3'-hydroxyl bearing carbon. The discovery of a selective crystallization of an N-silyl nucleoside intermediate enabled isolation of the desired β-anomer from the glycosylation step.
Kageyama M, Nagasawa T, Yoshida M, Ohrui H, Kuwahara S. Enantioselective total synthesis of the potent anti-HIV nucleoside EFdA. Org Lett. 2011 Oct 7;13(19):5264-6. doi: https://doi.org/10.1021/ol202116k. Epub 2011 Sep 2. PMID: 21888325.
A concise enantioselective total synthesis of 4'-ethynyl-2-fluoro-2'-deoxyadenosine (EFdA), an extremely potent anti-HIV agent, has been accomplished from (R)-glyceraldehyde acetonide in 18% overall yield by a 12-step sequence involving a highly diastereoselective ethynylation of an α-alkoxy ketone intermediate.
Fukuyama K, Ohrui H, Kuwahara S. Synthesis of EFdA via a diastereoselective aldol reaction of a protected 3-keto furanose. Org Lett. 2015 Feb 20;17(4):828-31. doi: https://doi.org/10.1021/ol5036535. Epub 2015 Feb 2. PMID: 25642994.
An efficient enantioselective total synthesis of EFdA, a remarkably potent anti-HIV nucleoside analogue with various favorable pharmacological profiles, has been achieved in 37% overall yield from diacetone-D-glucose by a 14-step sequence that features a highly diastereoselective installation of the tetrasubstituted stereogenic center at the C4' position, direct oxidative cleavage of an acetonide-protected diol derivative to an aldehyde, and one-pot 2'-deoxygenation of a ribonucleoside intermediate.
Kageyama M, Miyagi T, Yoshida M, Nagasawa T, Ohrui H, Kuwahara S. Concise synthesis of the anti-HIV nucleoside EFdA. Biosci Biotechnol Biochem. 2012;76(6):1219-25. doi: https://doi.org/10.1271/bbb.120134. Epub 2012 Jun 7. PMID: 22790950.
EFdA (4'-ethynyl-2-fluoro-2'-deoxyadenosine), a nucleoside reverse transcriptase inhibitor with extremely potent anti-HIV activity, was concisely synthesized from (R)-glyceraldehyde acetonide in an 18% overall yield by a 12-step sequence involving highly diastereoselective ethynylation of an α-alkoxy ketone intermediate. The present synthesis is superior, both in overall yield and in the number of steps, to the previous one which required 18 steps from an expensive starting material and resulted in a modest overall yield of 2.5%.
Kinvig, H., Cottura, N., Lloyd, A. et al. Evaluating Islatravir Administered Via Microneedle Array Patch for Long-Acting HIV Pre-exposure Prophylaxis Using Physiologically Based Pharmacokinetic Modelling. Eur J Drug Metab Pharmacokinet 47, 855–868 (2022). https://doi.org/10.1007/s13318-022-00793-6
Technologies for long-acting administration of antiretrovirals (ARVs) for the prevention and treatment of HIV are at the forefront of research initiatives aiming to tackle issues surrounding drug adherence with the current standard of once-daily oral administration. Islatravir (ISL) is an emerging ARV that shows promising characteristics for long-acting prevention and treatment both orally as well as through alternative routes of administration. Microneedle array patches (MAPs) are a pain-free and discreet transdermal delivery technology that offer extended-release administration of nanoparticulate drugs. This study aimed to utilise physiologically based pharmacokinetic (PBPK) modelling to predict the pharmacokinetics resulting from ISL administered via MAP and to identify key MAP characteristics required to sustain effective concentrations over extended dosing intervals.
Additional documents
No documents were uploaded
Useful links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Not provided