
|
Developed by 

|
Supported by 

|
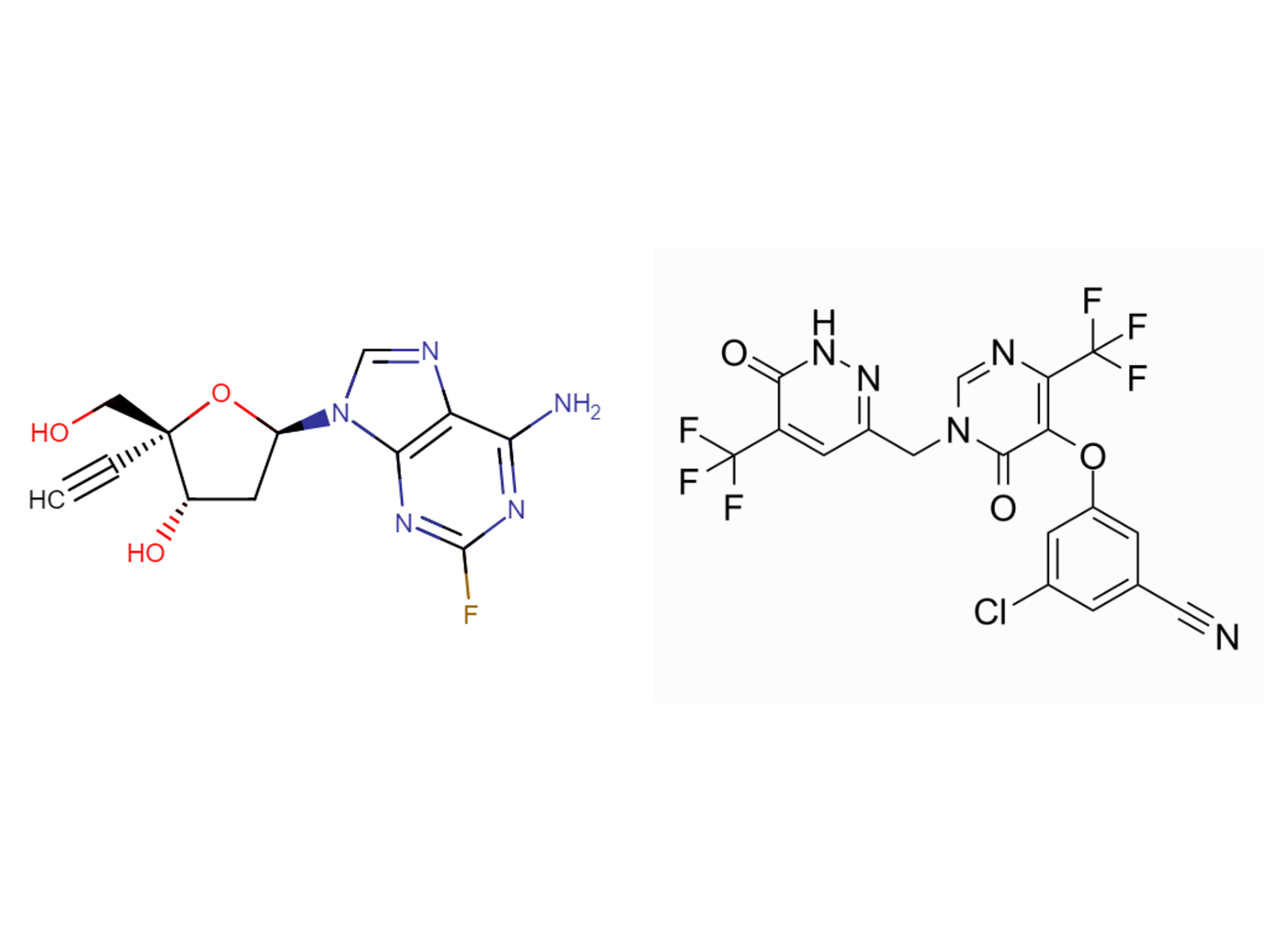
MK-8591B (islatravir + ulonivirine)
Developer(s)

|
Drug structure

islatravir and ulonivirine
Drug information
Associated long-acting platforms
Oral solid form
Administration route
Oral
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
User acceptance
Not provided
Dosage
Available dose and strength
Not provided
Frequency of administration
Not provided
Maximum dose
Not provided
Recommended dosing regimen
Not provided
Additional comments
Not provided
Dosage link(s)
Not provided
Drug information
Drug's link(s)
Not provided
Generic name
Brand name
Compound type
Summary
Approval status
Regulatory authorities
Delivery device(s)
No delivery device
Scale-up and manufacturing prospects
Scale-up prospects
Not provided
Tentative equipment list for manufacturing
Not provided
Manufacturing
Not provided
Specific analytical instrument required for characterization of formulation
Not provided
Clinical trials
8591-013
Identifier
NCT04564547
Link
https://clinicaltrials.gov/study/NCT04564547
Phase
Phase II
Status
Completed
Sponsor
Merck Sharp & Dohme LLC
More details
This is a randomized, controlled, double-blind, study to evaluate the safety and tolerability of islatravir (ISL) + ulonivirine based on review of the accumulated safety data, in adult participants with human immunodeficiency virus type 1 (HIV-1) who have been virologically suppressed for ≥6 months on bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) once-daily.
Purpose
Dose Ranging, Switch Study of Islatravir (ISL) and Ulonivirine (MK-8507) Once-Weekly in Virologically-Suppressed Adults With Human Immunodeficiency Virus Type 1 (HIV-1) [MK-8591-013]
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-03-09
Anticipated Date of Last Follow-up
2025-02-10
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2025-01-30
Actual Completion Date
2025-01-30
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Is HIV-1 positive with plasma HIV-1 RNA \<50 copies/mL at screening * Has been virologically suppressed on BIC/FTC/TAF for ≥6 months * Has a screening CD4+ T-cell count \>200 cells/mm\^3 (completed by the central laboratory) * Is male or female, at least 18 years of age, at the time of signing the informed consent * female participant is eligible to participate if she is not pregnant or breastfeeding, and at least one of the following conditions applies: * Is not a woman of childbearing potential (WOCBP) * Is a WOCBP and using a contraceptive method that is highly effective (with a failure rate of \<1% per year), or be abstinent from heterosexual intercourse as their preferred and usual lifestyle (abstinent on a long term and persistent basis) Exclusion Criteria: *
Health status
Study type
Interventional (clinical trial)
Enrollment
161
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
| Type of key results | Title | Website link |
|---|---|---|
| Article | Protocol Plain Language Summary | https://trialstransparency.merckclinicaltrials.com/Upload/1115_D1_PPLS_2024-511041-19_for%20pub_01May2024_V1-0_MK-8591-013-04.pdf |
8591B-060
Identifier
NCT06891066
Link
https://clinicaltrials.gov/study/NCT06891066
Phase
Phase II
Status
Recruiting
Sponsor
Merck Sharp & Dohme LLC
More details
Investigators are trying to find better treatments for people with HIV-1. In this clinical study, investigators want to see how well a new treatment called ISL+ULO, taken once a week, works compared to an existing treatment called BIC/FTC/TAF, which is taken every day. Investigators will check how many people still have a high level of the virus in their blood after 24 weeks. The investigators also want to understand if the new treatment, MK-8591B, is safe and how well people can handle it.
Purpose
A Study of Islatravir (ISL) and Ulonivirine (ULO) Once Weekly (QW) in Virologically Suppressed Adults With Human Immunodeficiency Virus Type 1 (HIV-1) (MK-8591B-060)
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2025-04-14
Anticipated Date of Last Follow-up
2025-05-10
Estimated Primary Completion Date
2027-09-24
Estimated Completion Date
2027-09-24
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion: The main inclusion criteria include but are not limited to the following: - Has been receiving Bictegravir/Emtricitabine/Tenofovir alafenamide (BIC/FTC/TAF) therapy with documented viral suppression \[Human immunodeficiency virus type 1 (HIV-1) ribonucleic acid (RNA) \<50 copies/mL\] for ≥6 months prior to providing documented informed consent and has no history of prior virologic treatment failure on any past or current regimen. Exclusion: The main exclusion criteria include but are not limited to the following: * Has Human immunodeficiency virus type 2 (HIV-2) infection. * Has a diagnosis of an active Acquired immune deficiency syndrome (AIDS)-defining opportunistic infection. * Has active hepatitis C virus (HCV) coinfection. * Has hepatitis B virus (HBV) coinfection. * H
Health status
Study type
Interventional (clinical trial)
Enrollment
150
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key results
Excipients
Proprietary excipients used
Not provided
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
Not provided
Residual solvents used
Not provided
Patent info
There are either no relevant patents or these were not yet submitted to LAPaL
Supporting material
Publications
Matthews RP, Patel M, Liu W, Liu Y, Rondón JC, Vargo RC, Stoch SA, Iwamoto M.2025.
Pharmacokinetics of islatravir in participants with moderate hepatic impairment.
Antimicrob Agents Chemother69:e01553-24.https://doi.org/10.1128/aac.01553-24
Islatravir (ISL) is a nucleoside reverse transcriptase translocation inhibitor in development for the treatment of HIV-1 infection. People living with HIV are at risk of liver disease. ISL is metabolized by adenosine deaminase (ADA), which is expressed in the liver; thus, ISL pharmacokinetics (PK) may be affected by hepatic impairment. This study evaluated the effect of moderate hepatic impairment on ISL PK. This nonrandomized, open-label, phase 1 study (MK-8591-030) evaluated the effects of a single oral dose of ISL 60 mg in HIV-seronegative adults with moderate hepatic insufficiency (n = 6) and matched healthy adult participants (n = 6). Blood samples for plasma ISL and 4′-ethynyl-2-fluoro-2′deoxyinosine (M4) and peripheral blood mononuclear cell (PBMC) ISL-triphosphate (ISL-TP) were collected at multiple time points through 672 h, and safety was monitored throughout. Modest decreases in maximum measured concentration (Cmax) and area under the concentration-time curve (AUC) of plasma ISL and AUC of PBMC ISL-TP were observed in participants with moderate hepatic impairment versus matched healthy participants, while ISL-TP Cmax was relatively unchanged. In contrast, plasma M4 was modestly increased in the moderate hepatic impairment group, suggesting that hepatic impairment may result in increased metabolism of ISL to M4 via ADA. The clinical relevance of the overall modest changes in M4, ISL, and ISL-TP levels with moderate hepatic impairment will be contextualized once exposure response data from ongoing clinical studies are available to elucidate the thresholds for clinical efficiency. A single oral dose of ISL 60 mg was generally well tolerated in both groups.
Additional documents
Useful links
There are no additional links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Not provided