
|
Developed by 

|
Supported by 

|
Paliperidone
Developer(s)

|
Janssen Pharmaceuticals https://www.janssen.com/Belgium Janssen Pharmaceuticals is a subsidiary company of Johnson & Johnson headquartered in Beerse, Belgium. They focus on manufacturing and developing pharmaceutical products for use in areas such as, Immunology, Infectious Diseases & Vaccines, Pulmonary Hypertension, Cardiovascular & Metabolism, Oncology, and Neuroscience. |
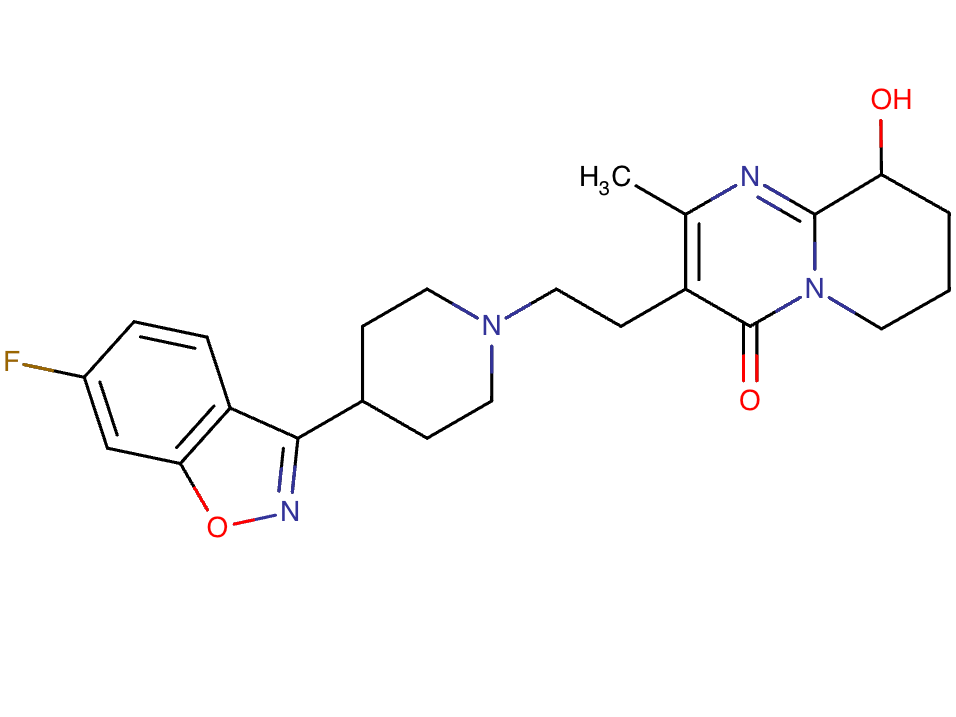
Drug structure

Paliperidone Chemical Structure
Sourced From DrugBank
Drug information
Associated long-acting platforms
Not provided
Administration route
Oral, Intramuscular
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
Frequency of administration
Not provided
User acceptance
Not provided
Dosage
Available dose and strength
Not provided
Maximum dose
Not provided
Recommended dosing regimen
Not provided
Additional comments
Not provided
Dosage link(s)
Not provided
Drug information
Drug's link(s)
Not provided
Generic name
Brand name
Compound type
Drug class/category
Summary
Approval status
Regulatory authorities
Delivery device(s)
Not provided
Scale-up and manufacturing prospects
Scale-up prospects
Not provided
Tentative equipment list for manufacturing
Not provided
Manufacturing
Not provided
Specific analytical instrument required for characterization of formulation
Not provided
Clinical trials
Not providedExcipients
Proprietary excipients used
Not provided
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
Not provided
Residual solvents used
Not provided
Patent info
Compound patent families
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Paliperidone dosing regimen
Expiry date: 2028-12-17 The present invention provides a method of treating patients in need of treatment with long acting injectable paliperidone palmitate formulations. |
WO2009080651 | Dose/Regimen | Janssen Pharmaceutica Nv | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Belarus, Azerbaijan, Moldova, Republic of, Armenia, Kazakhstan, Albania, Serbia, Bosnia and Herzegovina, Türkiye, North Macedonia, Ukraine, Malaysia, Philippines, Mexico, Sri Lanka, South Africa | Australia, Canada, Russian Federation, Liechtenstein, Italy, Norway, Malta, Denmark, Belgium, United Kingdom, Greece, Netherlands, Hungary, Croatia, Switzerland, Spain, Slovenia, Austria, Romania, Iceland, Cyprus, Finland, France, Bulgaria, Slovakia, Poland, Latvia, Ireland, Estonia, Germany, Luxembourg, Portugal, Czechia, Lithuania, Monaco, Sweden, Hong Kong, Japan, Korea, Republic of, New Zealand, United States of America, Singapore |
| Filed | Ecuador, Guatemala, Nicaragua, Indonesia | Finland, Hong Kong, Israel, Korea, Republic of |
| Not in force | World Intellectual Property Organization (WIPO), Brazil, China, Colombia, Tajikistan, Turkmenistan, Kyrgyzstan, Albania, Serbia, Bosnia and Herzegovina, Türkiye, North Macedonia, India | World Intellectual Property Organization (WIPO), Liechtenstein, Italy, Norway, Malta, Denmark, Belgium, United Kingdom, Greece, Netherlands, Hungary, Croatia, Switzerland, Spain, Slovenia, Austria, Romania, Iceland, Cyprus, Finland, France, Bulgaria, Slovakia, Poland, Latvia, Ireland, Estonia, Germany, Luxembourg, Portugal, Czechia, Lithuania, Monaco, Sweden, Israel, United States of America |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Paliperidone depot formulation for injection
Expiry date: 2018-11-10 The present invention is concerned with a pharmaceutical composition suitable as a depot formulation for administration via intramuscular or subcutaneous injection, comprising: (1) as an active ingredient a therapeutically effective amount of a 9-hydroxy-risperidone fatty acid ester or a salt, or a stereoisomer or a stereoisomeric mixture thereof in submicron form and (2) a pharmaceuticall acceptable carrier; wherein the pharmaceutically acceptable carrier is water and the active ingredient is suspended therein: and with a process of preparing such a composition. The invention further concerns such a pharmaceutical composition for use as a medicament in the treatment of psychosis, schizophrenia, schizoaffective disorders, non-schizophrenic psychoses, behavioural disturbances associated with neurodegenerative disorders, e.g. in dementia, behavioural disturbances in mental retardation and autism, Tourette's syndrome, bipolar mania, depression, anxiety. |
WO9925354 | Composition | Janssen Pharmaceutica N.V | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | ||
| Filed | ||
| Not in force | World Intellectual Property Organization (WIPO), Eswatini, Uganda, Zambia, Zimbabwe, Malawi, Ghana, Sudan, Botswana, Lesotho, Kenya, Gambia (the), Argentina, Brazil, China, Tajikistan, Belarus, Azerbaijan, Moldova, Republic of, Turkmenistan, Armenia, Kyrgyzstan, Kazakhstan, Albania, North Macedonia, Indonesia, Malaysia, Congo, Mauritania, Guinea-Bissau, Niger, Senegal, Cameroon, Mali, Togo, Burkina Faso, Benin, Côte d'Ivoire, Central African Republic, Guinea, Gabon, Chad, Türkiye, Ukraine, South Africa, Viet Nam, Mexico | World Intellectual Property Organization (WIPO), Australia, Bulgaria, Canada, Czechia, Russian Federation, Estonia, Liechtenstein, Italy, Denmark, Belgium, United Kingdom, Greece, Netherlands, Switzerland, Spain, Slovenia, Austria, Romania, Cyprus, Finland, France, Latvia, Ireland, Germany, Luxembourg, Portugal, Lithuania, Monaco, Sweden, Hong Kong, Croatia, Hungary, Israel, Japan, Korea, Republic of, Norway, New Zealand, Poland, Slovakia, United States of America, Singapore, Brunei Darussalam |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Paliperidone aqueous suspensions
Expiry date: 2017-05-12 The present invention is concerned with a pharmaceutical composition suitable as a depot formulation for administration via intramuscular or subcutaneous injection, comprising: (1) as an active ingredient a therapeutically effective amount of a 9-hydroxyrisperidone fatty acid ester or a salt, or a stereoisomer or a stereoisomeric mixture thereof and (2) a pharmaceutically acceptable carrier; wherein the pharmaceutically acceptable carrier is water and the active ingredient is suspended therein; and with a process of preparing such a composition. The invention further concerns such a pharmaceutical composition for use as a medicament in the treatment of schizophrenia, non-schizophrenic psychoses, behavioural disturbances associated with neurodegenerative disorders, e.g. in dementia, behavioural disturbances in mental retardation and autism, bipolar mania, depression, anxiety. |
WO9744039 | Composition | JANSSEN PHARMACEUTICA NV [BE] | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | ||
| Filed | ||
| Not in force | World Intellectual Property Organization (WIPO), Argentina, Brazil, China, Tajikistan, Belarus, Azerbaijan, Moldova, Republic of, Turkmenistan, Armenia, Kyrgyzstan, Kazakhstan, Albania, Indonesia, Mexico, Malaysia, Türkiye, Ukraine, South Africa | World Intellectual Property Organization (WIPO), Australia, Bulgaria, Canada, Cyprus, Czechia, Germany, Russian Federation, Estonia, Liechtenstein, Italy, Denmark, Belgium, United Kingdom, Greece, Netherlands, Switzerland, Spain, Slovenia, Austria, Romania, Finland, France, Latvia, Ireland, Luxembourg, Portugal, Lithuania, Monaco, Sweden, Hong Kong, Croatia, Hungary, Israel, Japan, Korea, Republic of, Norway, New Zealand, Poland, Slovakia, Taiwan, Province of China, United States of America |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Paliperidone compound and analogues and their use as antipsychotics
Expiry date: 2009-10-16 There is disclosed a process for preparing an enantiomeric form of the compound having the formula:or a pharmaceutically acceptable acid addition salt thereof,wherein said process comprises the steps of:(a) reacting a racemic mixture of said compound with a chiral acid or acid chloride selected from the group consisting of tartaric acid, malic acid, mandelic acid, camphor sulfonic acid, 4,5-dihydro-1H-2-benzopyran-2-carboxylic acid, and the acid chlorides thereof, to form a mixture of diastereomeric salts or esters;(b) physically separating said mixture of diastereomeric salts or esters by selective crystallization or chromatography; and(c) converting said separated diastereomeric salts or esters into the corresponding enantiomeric forms of said compound by hydrolysis in an acidic or basic aqueous medium. |
CA2000786 | Compound | Janssen Pharmaceutica, N. V | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | ||
| Filed | ||
| Not in force | South Africa | Canada, Australia, Chile, Cyprus, Germany, Denmark, Liechtenstein, Italy, Belgium, United Kingdom, Greece, Netherlands, Switzerland, Spain, Austria, France, Luxembourg, Sweden, Finland, Hong Kong, Ireland, Israel, Japan, Korea, Republic of, Norway, New Zealand, Portugal, United States of America |
Supporting material
Publications
There are no publication
Additional documents
No documents were uploaded
Useful links
There are no additional links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Not provided