
|
Developed by 

|
Supported by 

|
Rilpivirine Prodrug Nanoformulation (NM3RPV)
Developer(s)

|
University of Nebraska Medical Center Originator
https://www.unmc.edu/
United States The University of Nebraska Medical Center (UNMC) is a renowned public academic health science center located in Omaha, Nebraska. Established in 1881, UNMC is a leading institution in fields such as cancer research, infectious diseases, and rural health. The university is dedicated to advancing health care through innovative research, education, and patient care. |

|
Exavir Therapeutics Originator
https://exavirtherapeutics.com/
United States Exavir Therapeutics is a biopharmaceutical company focused on developing ultra-long-acting therapeutics for chronic viral infections and CNS disorders. Headquartered in San Francisco, CA, they utilize prodrug nano-formulation technology to extend the half-life of drugs. Their current research focus primarily targets HIV, with the goal of improving treatment adherence and patient outcomes. |
Drug structure

Rilpivirine Prodrug Nanoformulation (NM3RPV)
https://doi.org/10.1016/j.jconrel.2019.09.001
Drug information
Associated long-acting platforms
Aqueous drug particle suspension
Administration route
Intramuscular
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
User acceptance
Not provided
Dosage
Available dose and strength
Not yet available
Frequency of administration
Not yet available
Maximum dose
Not yet available
Recommended dosing regimen
Not yet available
Additional comments
Not provided
Dosage link(s)
Not provided
Drug information
Drug's link(s)
Not provided
Generic name
Brand name
Compound type
Summary
Approval status
Regulatory authorities
Delivery device(s)
No delivery device
Scale-up and manufacturing prospects
Scale-up prospects
The NM3RPV formulation is in preclinical development and has only been produced at small-scale for research use.
Tentative equipment list for manufacturing
Avestin EmulsiFlex-C3 high-pressure homogenizer, rotary evaporator, silica column chromatography. RPV and M3RPV samples were separated on a Phenomenex Kinetex 5 μm C18 column (150 × 4.6 mm) (Torrance, CA).
Manufacturing
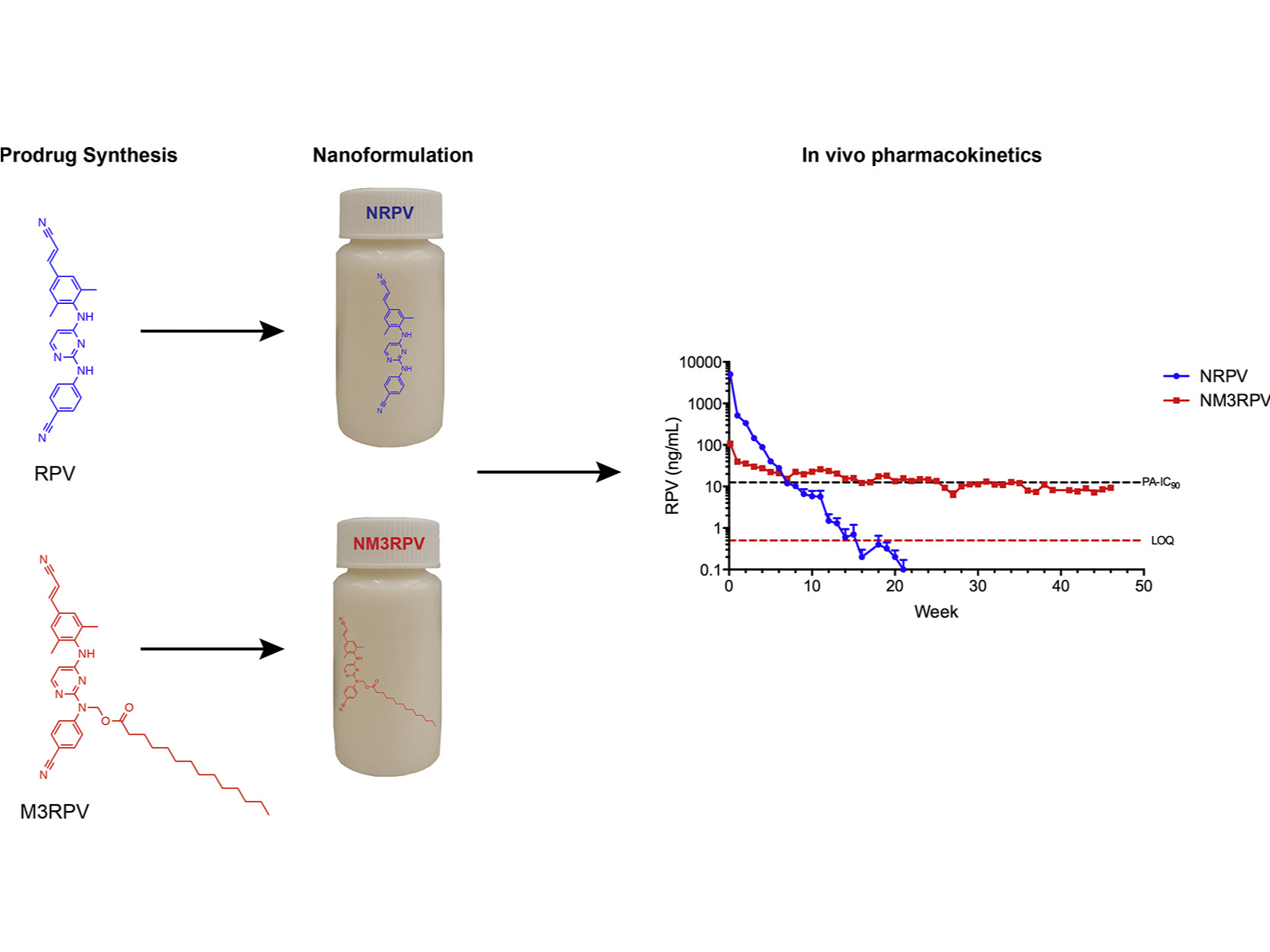
Nanoformulations of RPV and M3RPV (NRPV and NM3RPV, respectively) were manufactured using an Avestin EmulsiFlex-C3 high-pressure homogenizer (Ottawa, ON, Canada). NRPV was prepared to best replicate RPV LA developed by Janssen. Specifically, 1% (w/v) P338 was dispersed in H2O, followed by the addition of 10% (w/v) RPV-free base and mixing overnight. Similarly, NM3RPV contained 2.1% P407 and 21% M3RPV in H2O. Formulations were homogenized at 20,000 psi to generate homogeneous nanocrystals of uniform size and polydispersity.
Specific analytical instrument required for characterization of formulation
Nanoparticle physicochemical characterization for size (nm), PDI and zeta potential (mV) were evaluated by dynamic light scattering (DLS, Malvern Zetasizer Nano-ZS, Worcestershire, UK). NM3RPV and NRPV drug quantitation was conducted by UPLC-UV/Vis. Drug quantitation was performed on a Waters ACQUITY ultra-performance liquid chromatography (UPLC) H-Class system with TUV detector and Empower 3 software (Milford, MA).
Clinical trials
Not providedExcipients
Proprietary excipients used
Not provided
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
The novel excipient poloxamer 338 (P338) is used in the final RPV (NM3RPV) formulation. Following both an in-vitro mammalian chromosome aberration and an Ames test, it was considered to be non-genotoxic with no evidence for mutagenicity. Further P338 fertility, genotoxicity and development studies have been conducted with no negative effects, in addition to a 6-week and 9-month minipig repeat-dose toxicity study. No adverse local or systemic toxicity was reported in the minipigs at 100mg/month (Margin of Exposure:19).
Residual solvents used
Not provided
Patent info
There are either no relevant patents or these were not yet submitted to LAPaL
Supporting material
Publications
James R. Hilaire, Aditya N. Bade, Brady Sillman, Nagsen Gautam, Jonathan Herskovitz, Bhagya Laxmi Dyavar Shetty, Melinda S. Wojtkiewicz, Adam Szlachetka, Benjamin G. Lamberty, Sruthi Sravanam, Howard S. Fox, Yazen Alnouti, Prasanta K. Dash, JoEllyn M. McMillan, Benson J. Edagwa, Howard E. Gendelman, Creation of a long-acting rilpivirine prodrug nanoformulation, Journal of Controlled Release, Volumes 311–312, 2019, Pages 201-211, ISSN 0168-3659, DOI: https://doi.org/10.1016/j.jconrel.2019.09.001.(https://www.sciencedirect.com/science/article/pii/S0168365919305346)
Antiretroviral therapy requires lifelong daily dosing to attain viral suppression, restore immune function, and improve quality of life. As a treatment alternative, long-acting (LA) antiretrovirals can sustain therapeutic drug concentrations in blood for prolonged time periods. The success of recent clinical trials for LA parenteral cabotegravir and rilpivirine highlight the emergence of these new therapeutic options. Further optimization can improve dosing frequency, lower injection volumes, and facilitate drug-tissue distributions. To this end, we report the synthesis of a library of RPV prodrugs designed to sustain drug plasma concentrations and improved tissue biodistribution. The lead prodrug M3RPV was nanoformulated into the stable LA injectable NM3RPV. NM3RPV treatment led to RPV plasma concentrations above the protein-adjusted 90% inhibitory concentration for 25 weeks with substantial tissue depots after a single intramuscular injection in BALB/cJ mice. NM3RPV elicited 13- and 26-fold increases in the RPV apparent half-life and mean residence time compared to native drug formulation. Taken together, proof-of-concept is provided that nanoformulated RPV prodrugs can extend the apparent drug half-life and improve tissue biodistribution. These results warrant further development for human use.
Additional documents
No documents were uploaded
Useful links
There are no additional links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Not provided