
|
Developed by 

|
Supported by 

|
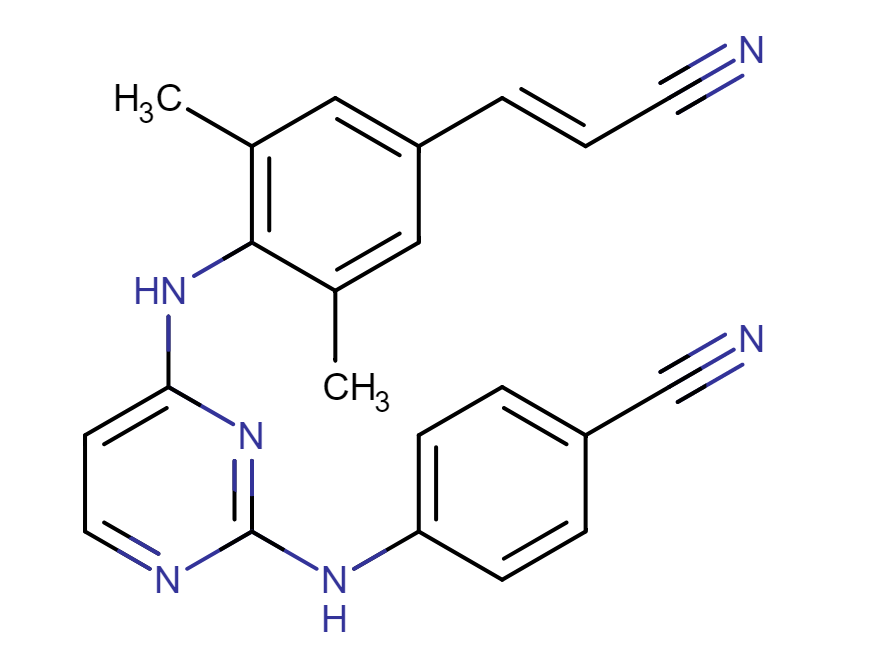
Rilpivirine (RPV)
Developer(s)

|
Janssen Pharmaceuticals Originator
https://www.janssen.com/
Belgium Janssen Pharmaceuticals is a subsidiary company of Johnson & Johnson headquartered in Beerse, Belgium. They manufacture and develop pharmaceutical products for use in areas such as, Immunology, Infectious Diseases & Vaccines, Pulmonary Hypertension, Cardiovascular & Metabolism, Oncology, and Neuroscience. |
Drug structure

Rilpivirine Chemical Structure
Sourced from Drugbank
Drug information
Associated long-acting platforms
Aqueous drug particle suspension
Administration route
Oral, Intramuscular
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
User acceptance
Not provided
Dosage
Available dose and strength
Not provided
Frequency of administration
Not provided
Maximum dose
Not provided
Recommended dosing regimen
Not provided
Additional comments
Not provided
Dosage link(s)
Not provided
Drug information
Drug's link(s)
Not provided
Generic name
Brand name
Compound type
Summary
Approval status
Regulatory authorities
Delivery device(s)
No delivery device
Scale-up and manufacturing prospects
Scale-up prospects
Compound is commercially manufactured.
Tentative equipment list for manufacturing
Netzsch ball mill, high pressure homogenizer.
Manufacturing
The manufacturing process for RPV is considered to be non-standard due to the inclusion of an aseptic processing step. RPV is light-sensitive, and exposure to light can induce conversion into a Z-isomer form which can affect pharmacokinetic data and activity. Therefore, all processing, manufacturing and storage stages must implement mitigation procedures and protection from light. The RPV nanosuspension is stored aseptically in single-use glass vials with a protective nitrogen atmosphere. The formulation requires refrigerated storage and is stable for up to three years at 5°C.
Specific analytical instrument required for characterization of formulation
Laser diffractor (determine particle size), FT-IR UHPLC (chemical identification), UHPLC (chromatographic purity), paddle apparatus & UPLC/UV (determine in-vitro drug release for QC / dissolution testing).
Clinical trials
HPTN 076
Identifier
NCT02165202
Link
https://clinicaltrials.gov/ct2/show/NCT02165202
Phase
Phase II
Status
Completed
Sponsor
PATH
More details
Not provided
Purpose
Comparing the safety of an intramuscular (IM) injection of TMC278 LA to a placebo given once every eight weeks over a 40 week period among sexually active, HIV- uninfected women.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2014-10-01
Anticipated Date of Last Follow-up
2018-07-27
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2015-04-13
Actual Completion Date
2017-03-09
Studied populations
Age Cohort
- Adults
Genders
- Cisgender female
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: - Women, 18- 45 years (inclusive) of age at Enrolment. - Female at birth. - Willing and able to provide informed consent to take part in the study, provide adequate locator information and acceptability and adherence assessments throughout the study. - Understands and agrees to local reporting requirements for sexually transmitted infections (STis). - No evidence of an active STI and no diagnosis of Chlamydia trachomatis (CT), Neisseria gonorrhoeae (GC), or syphilis within the last 6 months. - Availability to return for all study visits and participate in all study-related procedures. - Must agree to use condoms for the duration of the study. - Must agree not to participate in other concurrent drug or vaccine trials. - Normal laboratory values.
Health status
Study type
Interventional (clinical trial)
Enrollment
136
Allocation
Randomized
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Double-blind masking
Masking description
Double (Participant, Investigator)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
| Type of key results | Title | Website link |
|---|---|---|
| Article | Metabolism of Long-Acting Rilpivirine After Intramuscular Injection: HIV Prevention Trials Network Study 076 (HPTN 076) | https://doi.org/10.1089/aid.2020.0155 |
| Article | Acceptability of a long-acting injectable HIV prevention product among US and African women: findings from a phase 2 clinical Trial (HPTN 076) | https://doi.org/10.1002/jia2.25408 |
MWRI-01
Identifier
NCT01656018
Link
https://clinicaltrials.gov/ct2/show/NCT01656018
Phase
Phase I
Status
Completed
Sponsor
Janssen Research & Development, LLC
More details
Not provided
Purpose
Evaluate the safety, acceptability, pharmacokinetics, and ex vivo pharmacodynamics of long-acting TMC278 when administered as an intramuscular injection in HIV-1 negative adults.
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2012-11-01
Anticipated Date of Last Follow-up
2016-04-06
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2016-01-01
Actual Completion Date
2016-01-01
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: - Human immunodeficiency virus type 1 (HIV-1) seronegative at screening and enrolment. - Not pregnant or breastfeeding females. - Agrees to protocol-defined method of contraception. - Abstinence from insertion of anything in rectum (eg, medication, enema, penis, or sex toy) for 72 hours before and 72 hours after each rectal biopsy visit. - Abstinence from insertion of anything in vagina (eg, tampon, medication, douche, penis, or sex toy) for 72 hours before and 72 hours after each cervical and vaginal biopsy visit.
Health status
Study type
Interventional (clinical trial)
Enrollment
4
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
| Type of key results | Title | Website link |
|---|---|---|
| Article | Long-acting rilpivirine as potential pre-exposure prophylaxis for HIV-1 prevention (the MWRI-01 study): an open-label, phase 1, compartmental, pharmacokinetic and pharmacodynamic assessment | https://doi.org/10.1016/s2352-3018(16)30113-8 |
SSAT 040
Identifier
NCT01275443
Link
https://clinicaltrials.gov/ct2/show/NCT01275443
Phase
Phase I
Status
Completed
Sponsor
St Stephens Aids Trust
More details
Not provided
Purpose
Investigate the levels of the drug (TMC278LA) in the blood, tissues and fluids of the rectum, in addition to the safety and tolerability of the drug when given as a single dose.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2011-01-01
Anticipated Date of Last Follow-up
2017-06-23
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2012-06-01
Actual Completion Date
2012-06-01
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Up to 60 evaluable female participants will be enrolled, with more than 40% being of African ancestry. Six male participants will also be enrolled. Female participants will be non-pregnant, non-lactating, aged between 18 to 50 years, and possess a Body Mass Index (BMI) of 16 to 35 kg/m2, inclusive.
Health status
Study type
Interventional (clinical trial)
Enrollment
66
Allocation
Randomized
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
| Type of key results | Title | Website link |
|---|---|---|
| Article | A compartmental pharmacokinetic evaluation of long-acting rilpivirine in HIV-negative volunteers for pre-exposure prophylaxis | https://doi.org/10.1038/clpt.2014.118 |
CR016747
Identifier
NCT01031589
Link
https://clinicaltrials.gov/ct2/show/NCT01031589
Phase
Phase I
Status
Completed
Sponsor
Tibotec Pharmaceuticals, Ireland
More details
Not provided
Purpose
Investigate the safety/tolerability and pharmacokinetics of a Rilpivirine (RPV; TMC278) long-acting (LA) formulation after single and multiple intramuscular injections.
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2010-01-01
Anticipated Date of Last Follow-up
2012-11-19
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2011-07-01
Actual Completion Date
2011-07-01
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Participants required to be good health. Females were excluded from the trial unless they were postmenopausal for at least 2 years or surgically sterile.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
19
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Double-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
| Type of key results | Title | Website link |
|---|---|---|
| Article | Safety, tolerability and pharmacokinetics of rilpivirine following administration of a long-acting formulation in healthy volunteers | https://doi.org/10.1111/hiv.12247 |
CR108302
Identifier
NCT03127189
Link
https://clinicaltrials.gov/study/NCT03127189
Phase
Phase I
Status
Completed
Sponsor
Janssen Research & Development, LLC
More details
The main purpose of this study is to characterize the single-dose pharmacokinetics (PK) of rilpivirine (RPV) after intramuscular (IM) injection of rilpivirine long-acting parenteral formulation (RPV LA) nanosuspensions with different particle size distribution (PSD), in healthy adult participants.
Purpose
A Study to Investigate the Effect of Different Particle Sizes on the Single-dose Pharmacokinetics of Rilpivirine After Intramuscular Injection of a Long-acting Nanosuspension in Healthy Participants
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2017-04-20
Anticipated Date of Last Follow-up
2018-08-08
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2018-04-10
Actual Completion Date
2018-04-10
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: - Participant must be willing and able to adhere to the prohibitions and restrictions specified in this protocol. - Contraceptive use by men or women should be consistent with local regulations regarding the use of contraceptive methods for participants participating in clinical studies - A female participant of childbearing potential must have a negative serum beta-human chorionic gonadotropin test at screening and on Day -1 of each session - For the duration of the study and for at least 6 months after intramuscular (IM) injection of RPV LA (or 1 month after administration of rilpivirine (RPV) oral solution for participants who discontinue after Session 1), male and female participants must agree to practice effective contraception. - Participant must be non-smoking.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
110
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Unspecified
Key results
Excipients
Proprietary excipients used
No proprietary excipient used
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
The novel excipient poloxamer 338 (P338) is used in the final G001 clinical formulation. Following both an in-vitro mammalian chromosome aberration and an Ames test, it was considered to be non-genotoxic with no evidence for mutagenicity. Further P338 fertility, genotoxicity and development studies have been conducted with no negative effects, in addition to a 6-week and 9-month minipig repeat-dose toxicity study. No adverse local or systemic toxicity was reported in the minipigs at 100mg/month (Margin of Exposure:19).
Residual solvents used
No residual solvent used
Patent info
Compound patent families
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Aqueous suspensions of rilpivirine micro- or nanoparticles
Expiry date: 2027-06-22 This invention concerns pharmaceutical compositions for administration via intramuscular or subcutaneous injection, comprising micro- or nanoparticles of the NNRTI compound TMC278, suspended in an aqueous pharmaceutically acceptable carrier, and the use of such pharmaceutical compositions in the treatment and prophylaxis of HIV infection. |
WO2007147882 | Composition | Tibotec Pharmaceuticals Ltd | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Brazil, China, Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Moldova, Republic of, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Serbia, Mexico, Ukraine, India, Benin, Cameroon, Burkina Faso, Chad, Guinea-Bissau, Mali, Senegal, Congo, Guinea, Gabon, Niger, Equatorial Guinea, Mauritania, Togo, Côte d'Ivoire, Central African Republic, Jordan, Philippines, Thailand | Canada, Australia, Chile, Cyprus, Denmark, Russian Federation, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Monaco, Portugal, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Croatia, Romania, Latvia, Lithuania, Slovenia, Israel, Japan, Korea, Republic of, New Zealand, Singapore, United States of America, Bahrain, Kuwait, Qatar, Saudi Arabia, Oman, United Arab Emirates |
| Filed | Jordan, Pakistan, Venezuela (Bolivarian Republic of) | Cyprus, Denmark, Spain, Portugal, Finland, Hungary, Poland, Croatia, Lithuania, Slovenia, Taiwan, Province of China, Uruguay, Brunei Darussalam, Macao, Hong Kong |
| Not in force | Botswana, Gambia (the), Ghana, Kenya, Lesotho, Malawi, Mozambique, Namibia, Sierra Leone, Sudan, Eswatini, Tanzania, United Republic of, Uganda, Zambia, Zimbabwe, Argentina, Peru, World Intellectual Property Organization (WIPO), Indonesia, Egypt, South Africa, Viet Nam | United States of America, World Intellectual Property Organization (WIPO), Panama |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Rilpivine parenteral formulation
Expiry date: 2027-01-19 This invention relates to the use of a parenteral formulation comprising an anti-virally effective amount of TMC278 or a pharmaceutically acceptable acid-addition salt thereof, and a carrier, for the manufacture of a medicament for the treatment of a subject being infected with HIV, wherein the formulation is to be administered intermittently at a time interval of at least one week. |
WO2007082922 | Dose/Regimen, Use | Tibotec Pharmaceuticals Ltd | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Moldova, Republic of, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Serbia, Montenegro, Malaysia, Ukraine, Philippines, Thailand, Benin, Cameroon, Burkina Faso, Chad, Guinea-Bissau, Mali, Senegal, Congo, Guinea, Gabon, Niger, Equatorial Guinea, Mauritania, Togo, Côte d'Ivoire, Central African Republic, Mexico, Nigeria, South Africa | Canada, Australia, Russian Federation, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Croatia, Romania, Latvia, Lithuania, Slovenia, Hong Kong, Israel, Japan, Korea, Republic of, New Zealand, Singapore, Taiwan, Province of China, United States of America, Malta |
| Filed | Spain, Cyprus, Croatia, Hong Kong, Japan, Korea, Republic of, Singapore, United States of America | |
| Not in force | Botswana, Gambia (the), Ghana, Kenya, Lesotho, Malawi, Mozambique, Namibia, Sierra Leone, Sudan, Eswatini, Tanzania, United Republic of, Uganda, Zambia, Zimbabwe, Argentina, Brazil, China, World Intellectual Property Organization (WIPO), India, Egypt, Viet Nam, Indonesia | United States of America, World Intellectual Property Organization (WIPO), Chile |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Rilpivirine hydrochloride
Expiry date: 2025-09-02 The present invention relates to a pharmaceutical composition comprising as active ingredient the hydrochloric acid salt of rilpivirine and to processes for their preparation |
WO2006024668 | Salt | Copmans, Alex, Herman, Janssen Pharmaceutica N.V, Peeters, Jozef, Stappers, Alfred, Elisabeth, Stevens, Paul, Theodoor, Agnes, Tibotec Pharmaceuticals Ltd, Vandecruys, Roger, Petrus, Gerebern | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Mexico, Serbia, Botswana, Ghana, Gambia (the), Kenya, Lesotho, Malawi, Mozambique, Namibia, Sudan, Sierra Leone, Eswatini, Tanzania, United Republic of, Uganda, Zambia, Zimbabwe, Türkiye, Armenia, Azerbaijan, Belarus, Kyrgyzstan, Kazakhstan, Moldova, Republic of, Tajikistan, Turkmenistan, Indonesia, Philippines, Viet Nam, Ukraine, Montenegro, India, Albania, Bosnia and Herzegovina, North Macedonia, Congo, Mauritania, Guinea-Bissau, Niger, Senegal, Cameroon, Mali, Togo, Burkina Faso, Benin, Côte d'Ivoire, Central African Republic, Guinea, Gabon, Equatorial Guinea, Chad, South Africa, Sri Lanka | Austria, Australia, Canada, Spain, Israel, Japan, Korea, Republic of, Norway, New Zealand, Slovenia, Belgium, Bulgaria, Switzerland, Cyprus, Czechia, Germany, Denmark, Estonia, Finland, France, United Kingdom, Greece, Hungary, Ireland, Iceland, Italy, Liechtenstein, Lithuania, Luxembourg, Latvia, Monaco, Netherlands, Poland, Portugal, Romania, Sweden, Slovakia, Russian Federation, Croatia |
| Filed | Nicaragua, Egypt, Kosovo | Austria, Spain, Norway, Slovenia, France, Hungary, Lithuania, Hong Kong |
| Not in force | Brazil, China, Costa Rica, Ecuador, World Intellectual Property Organization (WIPO), Colombia | World Intellectual Property Organization (WIPO) |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Rilpivirine compound and analogues and their use in HIV
Expiry date: 2022-08-09 The present invention is concerned with pyrimidine derivatives having HIV (Human Immunodeficiency Virus) replication inhibiting properties. The invention further relates to methods for their preparation and pharmaceutical compositions comprising them. The invention also relates to the use of said compounds for the manufacture of a medicament for the prevention or the treatment of HIV infection. |
WO03016306 | Compound | Janssen Pharmaceutica N.V | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Brazil, Ukraine, Kazakhstan, Albania | Australia, Germany, Hungary, Israel, Japan, Korea, Republic of, Luxembourg, Norway, Poland, Slovenia, Taiwan, Province of China, United States of America, Austria, Belgium, Bulgaria, Switzerland, Cyprus, Czechia, Denmark, Estonia, Spain, Finland, France, Greece, Ireland, Italy, Liechtenstein, Monaco, Netherlands, Portugal, Sweden, Slovakia, Russian Federation, Chile, Romania, Latvia, Lithuania, Singapore |
| Filed | Venezuela (Bolivarian Republic of) | Hungary, Slovenia, Cyprus, France, Lithuania |
| Not in force | Argentina, Brazil, China, Egypt, Mexico, South Africa, Botswana, Ghana, Gambia (the), Kenya, Lesotho, Malawi, Mozambique, Sudan, Sierra Leone, Eswatini, Tanzania, United Republic of, Uganda, Zambia, Zimbabwe, Türkiye, Armenia, Azerbaijan, Belarus, Kyrgyzstan, Moldova, Republic of, Tajikistan, Turkmenistan, Burkina Faso, Benin, Central African Republic, Congo, Côte d'Ivoire, Cameroon, Gabon, Guinea, Equatorial Guinea, Guinea-Bissau, Mali, Mauritania, Niger, Senegal, Chad, Togo, India, Malaysia, Philippines, Thailand, Viet Nam, Sri Lanka, World Intellectual Property Organization (WIPO), Albania, North Macedonia, Jordan, Lebanon, Pakistan, Indonesia | Canada, Germany, Hong Kong, Croatia, Japan, Luxembourg, Norway, New Zealand, Panama, Poland, Slovenia, Taiwan, Province of China, United States of America, Austria, Belgium, Bulgaria, Switzerland, Cyprus, Czechia, Denmark, Estonia, Spain, Finland, France, United Kingdom, Greece, Ireland, Italy, Liechtenstein, Monaco, Netherlands, Portugal, Sweden, Slovakia, World Intellectual Property Organization (WIPO), Romania, Latvia, Lithuania, Kuwait, United Arab Emirates, Bahrain, Saudi Arabia, Oman, Qatar, Macao, Trinidad and Tobago |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Rilpivirine compound and analogues (Markush structure)
Expiry date: 2021-02-26 Pyrimidine derivatives of formula (I) wherein Q1, Q2, G and R<1> are as defined within; and pharmaceutically acceptable salts and in vivo hydrolysable esters thereof are described. Processes for their manufacture, pharmaceutical compositions and their use as cyclin-dependent serine/threonine kinase (CDK) and focal adhesion kinase (FAK) inhibitors are also described. |
WO0164656 | Compound | Astrazeneca Ab, Astrazeneca Uk Limited | No |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Austria, Belgium, Denmark, Finland, France, Greece, Ireland, Italy, Luxembourg, Netherlands, Sweden | |
| Filed | Norway, Cyprus, France | |
| Not in force | World Intellectual Property Organization (WIPO), Brazil, China, Mexico, South Africa, Türkiye, Albania, North Macedonia | Canada, United Kingdom, World Intellectual Property Organization (WIPO), Australia, Hong Kong, Israel, Japan, Korea, Republic of, Norway, New Zealand, United States of America, Switzerland, Cyprus, Germany, Spain, Liechtenstein, Monaco, Portugal, Romania, Latvia, Lithuania, Slovenia |
Supporting material
Publications
Jackson AG, Else LJ, Mesquita PM, et al. A compartmental pharmacokinetic evaluation of long-acting rilpivirine in HIV-negative volunteers for pre-exposure prophylaxis. Clin Pharmacol Ther. 2014;96(3):314-23. DOI: https://doi.org/10.1038/clpt.2014.118
Rilpivirine long-acting (RPV-LA) is a parenteral formulation enabling prolonged plasma exposure. We explored its multiple-compartment pharmacokinetics (PK) after a single dose, for pre-exposure prophylaxis. Sixty-six HIV-negative volunteers were enrolled: women received an intramuscular dose of 300, 600, or 1,200 mg, with plasma and genital levels measured to 84 days postdose; men receiving 600 mg had similar PK determined in plasma and rectum. Ex vivo antiviral activity of cervicovaginal lavage (CVL) was also assessed. After a single dose, RPV concentrations peaked at days 6-8 and were present in plasma and genital-tract fluid to day 84. Vaginal and male rectal tissue levels matched those in plasma. At the 1,200 mg dose, CVL showed greater antiviral activity, above baseline, at days 28 and 56. All doses were well tolerated. All doses gave prolonged plasma and genital-tract rilpivirine exposure. PK and viral inhibition of repeated doses will be important in further dose selection.
Mora-Peris B, Watson V, Vera JH, et al. Rilpivirine exposure in plasma and sanctuary site compartments after switching from nevirapine-containing combined antiretroviral therapy. J Antimicrob Chemother. 2014;69(6):1642-7. DOI: https://doi.org/10.1128/jvi.01912-19
As a long-acting formulation of the nonnucleoside reverse transcriptase inhibitor rilpivirine (RPV LA) has been proposed for use as preexposure prophylaxis (PrEP) and the prevalence of transmitted RPV-resistant viruses can be relatively high, we evaluated the efficacy of RPV LA to inhibit vaginal transmission of RPV-resistant HIV-1 in humanized mice. Vaginal challenges of wild-type (WT), Y181C, and Y181V HIV-1 were performed in mice left untreated or after RPV PrEP. Plasma viremia was measured for 7 to 10 weeks, and single-genome sequencing was performed on plasma HIV-1 RNA in mice infected during PrEP. RPV LA significantly prevented vaginal transmission of WT HIV-1 and Y181C HIV-1, which is 3-fold resistant to RPV. However, it did not prevent transmission of Y181V HIV-1, which has 30-fold RPV resistance in the viruses used for this study. RPV LA did delay WT HIV-1 dissemination in infected animals until genital and plasma RPV concentrations waned. Animals that became infected despite RPV LA PrEP did not acquire new RPV-resistant mutations above frequencies in untreated mice or untreated people living with HIV-1, and the mutations detected conferred low-level resistance. These data suggest that high, sustained concentrations of RPV were required to inhibit vaginal transmission of HIV-1 with little or no resistance to RPV but could not inhibit virus with high resistance. HIV-1 did not develop high-level or high-frequency RPV resistance in the majority of mice infected after RPV LA treatment. However, the impact of low-frequency RPV resistance on virologic outcome during subsequent antiretroviral therapy still is unclear.
IMPORTANCE The antiretroviral drug rilpivirine was developed into a long-acting formulation (RPV LA) to improve adherence for preexposure prophylaxis (PrEP) to prevent HIV-1 transmission. A concern is that RPV LA will not inhibit transmission of drug-resistant HIV-1 and may select for drug-resistant virus. In female humanized mice, we found that RPV LA inhibited vaginal transmission of WT or 3-fold RPV-resistant HIV-1 but not virus with 30-fold RPV resistance. In animals that became infected despite RPV LA PrEP, WT HIV-1 dissemination was delayed until genital and plasma RPV concentrations waned. RPV resistance was detected at similar low frequencies in untreated and PrEP-treated mice that became infected. These results indicate the importance of maintaining RPV at a sustained threshold after virus exposure to prevent dissemination of HIV-1 after vaginal infection and low-frequency resistance mutations conferred low-level resistance, suggesting that RPV resistance is difficult to develop after HIV-1 infection during RPV LA PrEP.
Ford N, Lee J, Andrieux-Meyer I, Calmy A: Safety, efficacy, and pharmacokinetics of rilpivirine: systematic review with an emphasis on resource-limited settings. HIV AIDS (Auckl). 2011;3:35-44. doi: https://doi.org/10.2147/hiv.s14559. Epub 2011 Apr 28.
The vast majority of people living with human immunodeficiency virus (HIV)/acquired immune deficiency syndrome reside in the developing world, in settings characterized by limited health budgets, critical shortages of doctors, limited laboratory monitoring, a substantial burden of HIV in children, and high rates of coinfection, in particular tuberculosis. Therefore, the extent to which new antiretrovirals will contribute to improvements in the management of HIV globally will depend to a large extent on their affordability, ease of use, low toxicity profile, availability as pediatric formulations, and compatibility with tuberculosis and other common drugs. We undertook a systematic review of the available evidence regarding drug interactions, and the efficacy and safety of rilpivirine (also known as TMC-278), and assessed our findings in view of the needs and constraints of resource-limited settings. The main pharmacokinetic interactions relevant to HIV management reported to date include reduced bioavailability of rilpivirine when coadministered with rifampicin, rifabutin or acid suppressing agents, and reduced bioavailability of ketoconazole. Potential recommendations for dose adjustment to compensate for these interactions have not been elaborated. Trials comparing rilpivirine and efavirenz found similar outcomes up to 96 weeks in intent-to-treat analysis; failure of rilpivirine was mainly virological, whereas failure among those exposed to efavirenz was mainly related to the occurrence of adverse events. Around half of the patients who fail rilpivirine develop non-nucleoside reverse transcriptase inhibitor resistance mutations. The incidence of Grade 2-4 events was lower for rilpivirine compared with efavirenz. Grade 3-4 adverse events potentially related to the drugs were infrequent and statistically similar for both drugs. No dose-response relationship was observed for efficacy or safety, and the lowest dose (25 mg) was selected for further clinical development. The potential low cost and dose of the active pharmaceutical ingredient means that rilpivirine can potentially be manufactured at a low price. Moreover, its long half-life suggests the potential for monthly dosing via nonoral routes, with promising early results from studies of a long-acting injectable formulation. These characteristics make rilpivirine an attractive drug for resource-limited settings. Future research should assess the potential to improve robustness and assess the clinical significance of interaction with antituberculosis drugs.
Azijn H, Tirry I, Vingerhoets J, de Bethune MP, Kraus G, Boven K, Jochmans D, Van Craenenbroeck E, Picchio G, Rimsky LT: TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother. 2010 Feb;54(2):718-27. doi: https://doi.org/10.1128/aac.00986-09. Epub 2009 Nov 23.
Nonnucleoside reverse transcriptase inhibitors (NNRTIs) have proven efficacy against human immunodeficiency virus type 1 (HIV-1). However, in the setting of incomplete viral suppression, efavirenz and nevirapine select for resistant viruses. The diarylpyrimidine etravirine has demonstrated durable efficacy for patients infected with NNRTI-resistant HIV-1. A screening strategy used to test NNRTI candidates from the same series as etravirine identified TMC278 (rilpivirine). TMC278 is an NNRTI showing subnanomolar 50% effective concentrations (EC50 values) against wild-type HIV-1 group M isolates (0.07 to 1.01 nM) and nanomolar EC50 values against group O isolates (2.88 to 8.45 nM). Sensitivity to TMC278 was not affected by the presence of most single NNRTI resistance-associated mutations (RAMs), including those at positions 100, 103, 106, 138, 179, 188, 190, 221, 230, and 236. The HIV-1 site-directed mutant with Y181C was sensitive to TMC278, whereas that with K101P or Y181I/V was resistant. In vitro, considerable cross-resistance between TMC278 and etravirine was observed. Sensitivity to TMC278 was observed for 62% of efavirenz- and/or nevirapine-resistant HIV-1 recombinant clinical isolates. TMC278 inhibited viral replication at concentrations at which first-generation NNRTIs could not suppress replication. The rates of selection of TMC278-resistant strains were comparable among HIV-1 group M subtypes. NNRTI RAMs emerging in HIV-1 under selective pressure from TMC278 included combinations of V90I, L100I, K101E, V106A/I, V108I, E138G/K/Q/R, V179F/I, Y181C/I, V189I, G190E, H221Y, F227C, and M230I/L. E138R was identified as a new NNRTI RAM. These in vitro analyses demonstrate that TMC278 is a potent next-generation NNRTI, with a high genetic barrier to resistance development.
Lade JM, Avery LB, Bumpus NN: Human biotransformation of the nonnucleoside reverse transcriptase inhibitor rilpivirine and a cross-species metabolism comparison. Antimicrob Agents Chemother. 2013 Oct;57(10):5067-79. doi: https://doi.org/10.1128/aac.01401-13. Epub 2013 Aug 5
Rilpivirine is a nonnucleoside reverse transcriptase inhibitor used to treat HIV-1. In the present study, the pathways responsible for the biotransformation of rilpivirine were defined. Using human liver microsomes, the formation of two mono- and two dioxygenated metabolites were detected via ultra high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS). Mass spectral analysis of the products suggested that these metabolites resulted from oxygenation of the 2,6-dimethylphenyl ring and methyl groups of rilpivirine. Chemical inhibition studies and cDNA-expressed cytochrome P450 (CYP) assays indicated that oxygenations were catalyzed primarily by CYP3A4 and CYP3A5. Glucuronide conjugates of rilpivirine and a monomethylhydroxylated metabolite of rilpivirine were also detected and were found to be formed by UDP-glucuronosyltransferases (UGTs) UGT1A4 and UGT1A1, respectively. All metabolites that were identified in vitro were detectable in vivo. Further, targeted UHPLC-MS/MS-based in vivo metabolomics screening revealed that rilpivirine treatment versus efavirenz treatment may result in differential levels of endogenous metabolites, including tyrosine, homocysteine, and adenosine. Rilpivirine biotransformation was also assessed across species using liver microsomes isolated from a range of mammals, and the metabolite profile identified using human liver microsomes was largely conserved for both oxidative and glucuronide metabolite formation. These studies provide novel insight into the metabolism of rilpivirine and the potential differential effects of rilpivirine- and efavirenz-containing antiretroviral regimens on the endogenous metabolome.
Van Klooster G. Hoeben E. Borghys H. et al. Pharmacokinetics and Disposition of Rilpivirine (TMC278) Nanosuspension as a long-acting injectable antiretroviral formulation. Antimicrob Agents Chemother. 2010; 54 (50066-4804/10/$12.00): 2042-2050. doi: https://doi.org/10.1128/AAC.01529-09
The next-generation human immunodeficiency virus type 1 (HIV-1) nonnucleoside reverse transcriptase inhibitor rilpivirine (TMC278) was administered in rats and dogs as single intramuscular (IM) or subcutaneous (SC) injections, formulated as a 200-nm nanosuspension. The plasma pharmacokinetics, injection site concentrations, disposition to lymphoid tissues, and tolerability were evaluated in support of its potential use as a once-monthly antiretroviral agent in humans. Rilpivirine plasma concentration-time profiles showed sustained and dose-proportional release over 2 months in rats and over 6 months in dogs. The absolute bioavailability approached 100%, indicating a complete release from the depot, in spite of rilpivirine concentrations still being high at the injection site(s) 3 months after administration in dogs. For both species, IM administration was associated with higher initial peak plasma concentrations and a more rapid washout than SC administration, which resulted in a stable plasma-concentration profile over at least 6 weeks in dogs. The rilpivirine concentrations in the lymph nodes draining the IM injection site exceeded the plasma concentrations by over 100-fold 1 month after administration, while the concentrations in the lymphoid tissues decreased to 3- to 6-fold the plasma concentrations beyond 3 months. These observations suggest uptake of nanoparticles by macrophages, which generates secondary depots in these lymph nodes. Both SC and IM injections were generally well tolerated and safe, with observations of a transient inflammatory response at the injection site. The findings support clinical investigations of rilpivirine nanosuspension as a long-acting formulation to improve adherence during antiretroviral therapy and for preexposure prophylaxis.
Mc Crudden MTC, Larrañeta E, Clark A et al. Design, formulation and evaluation of novel dissolving microarray patches containing a long-acting rilpivirine nanosuspension. J Control Release 2018; 292: 119–29. doi: https://doi.org/10.1016/j.jconrel.2018.11.002
One means of combating the spread of human immunodeficiency virus (HIV) is through the delivery of long-acting, antiretroviral (ARV) drugs for prevention and treatment. The development of a discreet, self-administered and self-disabling delivery vehicle to deliver such ARV drugs could obviate compliance issues with daily oral regimens. Alternatives in development, such as long-acting intramuscular (IM) injections, require regular access to health care facilities and disposal facilities for sharps. Consequently, this proof of concept study was developed to evaluate the use of dissolving microarray patches (MAPs) containing a long-acting (LA) nanosuspension of the candidate ARV drug, rilpivirine (RPV). MAPs were mechanically strong and penetrated skin in vitro, delivering RPV intradermally. In in vivo studies, the mean plasma concentration of RPV in rats (431 ng/ml at the Day 7 time point) was approximately ten-fold greater than the trough concentration observed after a single-dose in previous clinical studies. These results are the first to indicate, by the determination of relative exposures between IM and MAP administration, that larger multi-array dissolving MAPs could potentially be used to effectively deliver human doses of RPV LA. Importantly, RPV was also detected in the lymph nodes, indicating the potential to deliver this ARV agent into one of the primary sites of HIV replication over extended durations. These MAPs could potentially improve patient acceptability and adherence to HIV prevention and treatment regimens and combat instances of needle-stick injury and the transmission of blood-borne diseases, which would have far-reaching benefits, particularly to those in the developing world.
Additional documents
No documents were uploaded
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Not provided