
|
Developed by 

|
Supported by 

|
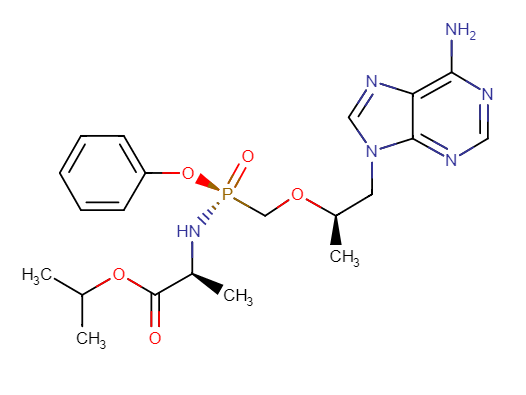
Tenofovir alafenamide (TAF)
Developer(s)

|
Gilead Sciences Inc. https://www.gilead.com/United States Gilead Sciences, Inc. is a multinational biopharmaceutical company that develops and manufactures innovative medicines for life-threatening diseases, including anti-viral therapeutics for HIV/AIDS, Hepatitis B, Hepatitis C and Covid-19. Headquartered in Foster City, California, Gilead was originally founded in 1987 and is currently listed on both the S&P 500 and the NASDAQ Biotechnology Index. |
Drug structure

Tenofovir Alafenamide Chemical Structure
Sourced from DrugBank
Drug information
Associated long-acting platforms
Polymeric implant, Titanium implant
Administration route
Oral, Subcutaneous
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
Frequency of administration
Not provided
User acceptance
Not provided
Dosage
Available dose and strength
Not provided
Maximum dose
Not provided
Recommended dosing regimen
Not provided
Additional comments
Not provided
Dosage link(s)
Not provided
Drug information
Drug's link(s)
Generic name
Brand name
Compound type
Drug class/category
Summary
Approval status
Regulatory authorities
Delivery device(s)
Several pre-clinical implant devices are currently in development for Tenofovir Alafenamide HIV PrEP. These include but are not limited to; [1] OCIS-001 consisting of a permeable polyurethane membrane developed by Northwestern University currently in Phase I/II trials, [2] Titanium reservoir containing nanochannels housed in a silicon membrane created by the Houston Methodist Research Institute, [3] Non-permeable silicone reservoir implant from the Oak Crest Institute of Science, and [4] a biodegradable poly(ε-caprolactone) reservoir implant developed by RTI International.
Scale-up and manufacturing prospects
Scale-up prospects
Not provided
Tentative equipment list for manufacturing
Not provided
Manufacturing
Not provided
Specific analytical instrument required for characterization of formulation
Thermal stability characterization via thermogravimetric analysis and differential scanning calorimetry to the implantable, dissolving and/or pure drug formulations. Drug crystallinity can be tested using a powder diffractometer (e.g. Miniflex X-ray from Rigaku Coporation). Drug stability and quantification are determined using HPLC (e.g. SphereClone™ C18 ODS (length, 150 mm; particle size, 5 μm; pore size, 80 Å; internal diameter, 4.6 mm). In vitro drug release studied via dialysis membrane bags (e.g. Spectra-Por®, 12,000–14,000 MWCO, Spectrum Medical Industries, Los Angeles, CA, USA).
Clinical trials
CAPRISA 018
Identifier
PACTR201809520959443
Link
https://pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=3584
Phase
Phase I/II
Status
Suspended
Sponsor
Centre for the AIDS Programme of Research in South Africa
More details
Status: Suspended (Pending review of the pharmacokinetic and safety data)
Purpose
Prevention
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2020-02-27
Actual Start Date
2020-07-09
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
2023-12-31
Estimated Completion Date
2023-12-31
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
Genders
- Cisgender female
- Transgender female
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Female (Phase I: age 18-40, Phase II: age 18-30). Phase I participants must be at low risk of HIV infection. Participants are excluded from the study if they are currently taking tenofovir-containing oral PrEP drugs, pregnant or breastfeeding.
Health status
Study type
Interventional (clinical trial)
Enrollment
550
Allocation
Randomized
Intervention model
Factorial assignment
Intervention model description
Not provided
Masking
Double-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key resources
| Type | Title | Content | Link |
|---|---|---|---|
| Link | CAPRISA 018: a phase I/II clinical trial study protocol to assess the safety, acceptability, tolerability and pharmacokinetics of a sustained-release tenofovir alafenamide subdermal implant for HIV | http://dx.doi.org/10.1136/bmjopen-2021-052880 |
Excipients
Proprietary excipients used
Not provided
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
Not provided
Residual solvents used
Not provided
Patent info
Compound patent families
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
TAF manufacturing process
Expiry date: 2032-10-03 Methods for isolating 9-{(R)-2-[((S)-{[(S)-l - (isopropoxycarbonyl)ethyl]amino}phenoxyphosphinyl)methoxy]propyl}adenine:'' (compound 16): a method for preparing, in high diastereomeric purity, intermediate compounds 13 and 15: method for preparing intermediate compound 12: 9-{(R)-2-[((S)-{[(S)-l - (isopropoxycarbonyl)ethyl]amino}phenoxyphosphinyl)methoxy]propyl}adenine has anti-viral properties. |
WO2013052094 | Process | Gilead Sciences, Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | China, Colombia, Mexico, Armenia, Azerbaijan, Belarus, Kyrgyzstan, Kazakhstan, Tajikistan, Turkmenistan, Türkiye, Brazil, Montenegro, India, Bolivia (Plurinational State of) | Australia, Canada, Hong Kong, Japan, Korea, Republic of, Taiwan, Province of China, United States of America, Russian Federation, Austria, Belgium, Switzerland, Czechia, Germany, Spain, France, United Kingdom, Greece, Ireland, Italy, Liechtenstein, Netherlands, Norway, Poland, Portugal, Sweden, Slovenia, Slovakia, New Zealand, Israel, Bahamas, Macao |
| Filed | China | Hong Kong, Korea, Republic of |
| Not in force | Argentina, Peru, Albania, North Macedonia, Serbia, Türkiye, World Intellectual Property Organization (WIPO), Brazil, Bosnia and Herzegovina, Montenegro, Ecuador, El Salvador, Pakistan, Egypt, Paraguay, Venezuela (Bolivarian Republic of) | Chile, Costa Rica, Japan, Uruguay, Bulgaria, Cyprus, Czechia, Denmark, Estonia, Finland, Greece, Croatia, Hungary, Iceland, Lithuania, Luxembourg, Latvia, Monaco, Malta, Norway, Poland, Romania, Slovenia, Slovakia, San Marino, World Intellectual Property Organization (WIPO), Kuwait, United Arab Emirates, Bahrain, Saudi Arabia, Oman, Qatar, Panama |
MPP Licence(s)
MPP licence on tenofovir alafenamide (TAF)
http://www.medicinespatentpool.org/licence-post/tenofovir-alafenamide-taf/Bilateral licences on TAF and TDF
https://www.gilead.com/purpose/medication-access/global-access/access-partnershipsPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Tenofovir alafenamide hemifumarate (TAF)
Expiry date: 2032-08-15 A hemifumarate form of tenofovir alafenamide, and antiviral therapy using tenofovir alafenamide hemifurnarate (e.g., anti-HTV and anti-HBV therapies). |
WO2013025788 | Salt | Gilead Sciences, Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Morocco, Moldova, Republic of, Mexico, Peru, Botswana, Ghana, Gambia (the), Kenya, Liberia, Lesotho, Malawi, Mozambique, Namibia, Rwanda, Sudan, Sierra Leone, Eswatini, Tanzania, United Republic of, Uganda, Zambia, Zimbabwe, Armenia, Azerbaijan, Belarus, Kyrgyzstan, Kazakhstan, Tajikistan, Turkmenistan, Viet Nam, Benin, Cameroon, Burkina Faso, Chad, Guinea-Bissau, Mali, Senegal, Congo, Guinea, Gabon, Niger, Equatorial Guinea, Mauritania, Togo, Côte d'Ivoire, Central African Republic, Bolivia (Plurinational State of), Philippines, South Africa, Ukraine, Brazil, El Salvador, Montenegro, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Serbia | United States of America, Australia, Canada, Chile, Costa Rica, Hong Kong, Israel, Japan, Korea, Republic of, New Zealand, Singapore, Taiwan, Province of China, Uruguay, Russian Federation, Denmark, Panama, Croatia, Cyprus, Bahamas, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Monaco, Portugal, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Romania, Latvia, Lithuania, Slovenia |
| Filed | China, Ecuador, India, Thailand, Venezuela (Bolivarian Republic of), Türkiye, North Macedonia, Albania, Serbia, Egypt | Hong Kong, Denmark, Bahrain, Kuwait, Qatar, Saudi Arabia, Oman, United Arab Emirates, Croatia, Cyprus, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Monaco, Portugal, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Romania, Latvia, Lithuania, Slovenia |
| Not in force | World Intellectual Property Organization (WIPO), Argentina, China, Colombia, Indonesia, Pakistan, Paraguay, Brazil, Montenegro, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Serbia | World Intellectual Property Organization (WIPO), Hong Kong, Israel, Japan, New Zealand, Denmark, Croatia, Cyprus, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Monaco, Portugal, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Romania, Latvia, Lithuania, Slovenia |
MPP Licence(s)
MPP licence on tenofovir alafenamide (TAF)
http://www.medicinespatentpool.org/licence-post/tenofovir-alafenamide-taf/Bilateral licences on TAF and TDF
https://www.gilead.com/purpose/medication-access/global-access/access-partnershipsPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Tenofovir alafenamide fumarate (TAF)
Expiry date: 2021-07-20 A novel method is provided for screening prodrugs of methoxyphosphonate nucleotide analogues to identify prodrugs selectively targeting desired tissues with antiviral or antitumor activity. This method has led to the identification of novel mixed ester-amidates of PMPA for retroviral or hepadnaviral therapy, including compounds of structure (5a) having substituent groups as defined herein. Compositions of these novel compounds in pharmaceutically acceptable excipients and their use in therapy and prophylaxis are provided. Also provided is an improved method for the use of magnesium alkoxide for the preparation of starting materials and compounds for use herein. |
WO0208241 | Compound | Gilead Sciences, Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Ukraine, Albania, Ethiopia, Fiji, Grenada, Kiribati, Solomon Islands, Saint Lucia | Australia, Bulgaria, Croatia, Israel, Iceland, Japan, Korea, Republic of, Norway, Poland, Russian Federation, Austria, Belgium, Switzerland, Cyprus, Germany, Denmark, Spain, Finland, France, United Kingdom, Greece, Ireland, Italy, Liechtenstein, Luxembourg, Netherlands, Sweden, Lithuania, Romania, Latvia, Slovenia, Estonia, Brunei Darussalam, Czechia, Anguilla, Bermuda, Falkland Islands (Malvinas), Montserrat, Turks and Caicos Islands, Virgin Islands (British), Cayman Islands, Gibraltar, Guernsey, Hungary |
| Filed | Jamaica | Norway |
| Not in force | China, Mexico, Türkiye, South Africa, Ghana, Gambia (the), Kenya, Lesotho, Malawi, Mozambique, Sudan, Sierra Leone, Eswatini, Tanzania, United Republic of, Uganda, Zimbabwe, Armenia, Azerbaijan, Belarus, Kyrgyzstan, Kazakhstan, Moldova, Republic of, Tajikistan, Turkmenistan, Burkina Faso, Benin, Central African Republic, Congo, Côte d'Ivoire, Cameroon, Gabon, Guinea, Equatorial Guinea, Guinea-Bissau, Mali, Mauritania, Niger, Senegal, Chad, Togo, India, Indonesia, Viet Nam, World Intellectual Property Organization (WIPO), North Macedonia, Albania, Congo, democratic Republic of the, Haiti, Nepal, Tuvalu, Brazil | Canada, Australia, Hong Kong, Croatia, Japan, Korea, Republic of, Norway, New Zealand, United States of America, Austria, Belgium, Switzerland, Cyprus, Germany, Denmark, Spain, Finland, France, United Kingdom, Greece, Ireland, Italy, Liechtenstein, Luxembourg, Monaco, Netherlands, Portugal, Sweden, World Intellectual Property Organization (WIPO), Lithuania, Romania, Latvia, Slovenia, Czechia, Guyana, Seychelles, Saint Helena, Ascension and Tristan da Cunha, Singapore, Jersey |
MPP Licence(s)
MPP licence on tenofovir alafenamide (TAF)
http://www.medicinespatentpool.org/licence-post/tenofovir-alafenamide-taf/Bilateral licences on TAF and TDF
https://www.gilead.com/purpose/medication-access/global-access/access-partnershipsSupporting material
Publications
Gunawardana, M., Remedios-Chan, M., Sanchez, D. et al. Fundamental aspects of long-acting tenofovir alafenamide delivery from subdermal implants for HIV prophylaxis. Sci Rep 12, 8224 (2022). https://doi.org/10.1038/s41598-022-11020-2
Global efforts aimed at preventing human immunodeficiency virus type one (HIV-1) infection in vulnerable populations appear to be stalling, limiting our ability to control the epidemic. Long-acting, controlled drug administration from subdermal implants holds significant potential by reducing the compliance burden associated with frequent dosing. We, and others, are exploring the development of complementary subdermal implant technologies delivering the potent prodrug, tenofovir alafenamide (TAF). The current report addresses knowledge gaps in the preclinical pharmacology of long-acting, subdermal TAF delivery using several mouse models. Systemic drug disposition during TAF implant dosing was explained by a multi-compartment pharmacokinetic (PK) model. Imaging mass spectrometry was employed to characterize the spatial distribution of TAF and its principal five metabolites in local tissues surrounding the implant. Humanized mouse studies determined the effective TAF dose for preventing vaginal and rectal HIV-1 acquisition. Our results represent an important step in the development of a safe and effective TAF implant for HIV-1 prevention.
Li, Linying, Leah M. Johnson, Sai Archana Krovi, Zach R. Demkovich, and Ariane van der Straten. 2020. "Performance and Stability of Tenofovir Alafenamide Formulations within Subcutaneous Biodegradable Implants for HIV Pre-Exposure Prophylaxis (PrEP)" Pharmaceutics 12, no. 11: 1057. https://doi.org/10.3390/pharmaceutics12111057
A critical need exists to develop diverse biomedical strategies for the widespread use of HIV Pre-Exposure Prophylaxis (HIV PrEP). This manuscript describes a subcutaneous reservoir-style implant for long-acting delivery of tenofovir alafenamide (TAF) for HIV PrEP. We detail key parameters of the TAF formulation that affect implant performance, including TAF ionization form, the selection of excipient and the exposure to aqueous conditions. Both in-vitro studies and shelf stability tests demonstrate enhanced performance for TAF freebase (TAFFB) in this long-acting implant platform, as TAFFB maintains higher chemical stability than the TAF hemifumarate salt (TAFHF). We also examined the hydrolytic degradation profiles of various formulations of TAF and identified inflection points for the onset of the accelerated drug hydrolysis within the implant using a two-line model. The compositions of unstable formulations are characterized by liquid chromatography-mass spectrometry (LC-MS) and are correlated to predominant products of the TAF hydrolytic pathways. The hydrolysis rate of TAF is affected by pH and water content in the implant microenvironment. We further demonstrate the ability to substantially delay the degradation of TAF by reducing the rates of drug release and thus lowering the water ingress rate. Using this approach, we achieved sustained release of TAFFB formulations over 240 days and maintained > 93% TAF purity under simulated physiological conditions. The opportunities for optimization of TAF formulations in this biodegradable implant supports further advancement of strategies to address long-acting HIV PrEP.
Vivek Agrahari, Sharon M. Anderson, M. Melissa Peet, Andrew P. Wong, Onkar N. Singh, Gustavo F. Doncel & Meredith R. Clark (2022) Long-acting HIV pre-exposure prophylaxis (PrEP) approaches: recent advances, emerging technologies, and development challenges, Expert Opinion on Drug Delivery, 19:10, 1365-1380, DOI: https://doi.org/10.1080/17425247.2022.2135699
Introduction: Poor or inconsistent adherence to daily oral pre-exposure prophylaxis (PrEP) has emerged as a key barrier to effective HIV prevention. The advent of potent long-acting (LA) antiretrovirals (ARVs) in conjunction with advances in controlled release technologies has enabled LA ARV drug delivery systems (DDS) capable of providing extended dosing intervals and overcome the challenge of suboptimal drug adherence with daily oral dosing.
Areas covered: This review discusses the current state of the LA PrEP field, recent advances, and emerging technologies, including ARV prodrug modifications and new DDS. Technological challenges, knowledge gaps, preclinical testing considerations, and future directions important in the context of clinical translation and implementation of LA HIV PrEP are discussed.
Expert opinion: The HIV prevention field is evolving faster than ever and the bar for developing next-generation LA HIV prevention options continues to rise. The requirements for viable LA PrEP products to be implemented in resource-limited settings are challenging, necessitating proactive consideration and product modifications during the design and testing of promising new candidates. If successfully translated, next-generation LA PrEP that are safe, affordable, highly effective, and accepted by both end-users and key stakeholders will offer significant potential to curb the HIV pandemic.
Romano JW, Baum MM, Demkovich ZR, Diana F, Dobard C, Feldman PL, Garcia-Lerma JG, Grattoni A, Gunawardana M, Ho DK, Hope TJ, Massud I, Milad M, Moss JA, Pons-Faudoa FP, Roller S, van der Straten A, Srinivasan S, Veazey RS, Zane D. Tenofovir Alafenamide for HIV Prevention: Review of the Proceedings from the Gates Foundation Long-Acting TAF Product Development Meeting. AIDS Res Hum Retroviruses. 2021 Jun;37(6):409-420. doi: https://doi.org/10.1089/aid.2021.0028. PMID: 33913760; PMCID: PMC8213003.
The ability to successfully develop a safe and effective vaccine for the prevention of HIV infection has proven challenging. Consequently, alternative approaches to HIV infection prevention have been pursued, and there have been a number of successes with differing levels of efficacy. At present, only two oral preexposure prophylaxis (PrEP) products are available, Truvada and Descovy. Descovy is a newer product not yet indicated in individuals at risk of HIV-1 infection from receptive vaginal sex, because it still needs to be evaluated in this population. A topical dapivirine vaginal ring is currently under regulatory review, and a long-acting (LA) injectable cabotegravir product shows strong promise. Although demonstrably effective, daily oral PrEP presents adherence challenges for many users, particularly adolescent girls and young women, key target populations. This limitation has triggered development efforts in LA HIV prevention options. This article reviews efforts supported by the Bill & Melinda Gates Foundation, as well as similar work by other groups, to identify and develop optimal LA HIV prevention products. Specifically, this article is a summary review of a meeting convened by the foundation in early 2020 that focused on the development of LA products designed for extended delivery of tenofovir alafenamide (TAF) for HIV prevention. The review broadly serves as technical guidance for preclinical development of LA HIV prevention products. The meeting examined the technical feasibility of multiple delivery technologies, in vivo pharmacokinetics, and safety of subcutaneous (SC) delivery of TAF in animal models. Ultimately, the foundation concluded that there are technologies available for long-term delivery of TAF. However, because of potentially limited efficacy and possible toxicity issues with SC delivery, the foundation will not continue investing in the development of LA, SC delivery of TAF products for HIV prevention.
Ying Jiang, Xinyi Gao, Onkar N. Singh, Wei Zhang, Vivek Agrahari, M. Melissa Peet, Meredith R. Clark, Gustavo F. Doncel, Ajay K. Banga, Pharmacokinetics of a weekly transdermal delivery system of tenofovir alafenamide in hairless rats, International Journal of Pharmaceutics, Volume 582, 2020, https://doi.org/10.1016/j.ijpharm.2020.119342.
Tenofovir alafenamide (TAF) is a potent prodrug of tenofovir (TFV) for HIV prophylaxis, and HIV and HBV treatment. Compared to oral daily doses, transdermal administration of TAF may be more advantageous for long-term adherence by offering sustained drug delivery and reduced dosing frequency. Here, we described the plasma pharmacokinetics (PK) of an optimized once-weekly suspension transdermal delivery system (TDS) for TAF (96 mg/25 cm2 of TDS) in female hairless rats. Over the study period, the TAF TDS delivered an overall low level of TAF (median: 1.43 [0.02–3.28] ng/mL) and a sustained level of the stable metabolite and parent drug, TFV. Relative to the projected exposure corresponding to six-day oral daily doses, a comparable TAF exposure but a substantially lower TFV exposure was resulted from the TAF TDS, suggesting a lower risk of TFV-associated adverse effects. TAF, TFV, and phosphorylated TFVs (TFV-monophosphate and diphosphate) were found distributed in vaginal tissue, the portal of entry for HIV during male-to-female sexual transmission. Skin adhesion and tolerance were acceptable given the animal model used. PK evaluation of the TAF TDS in hairless rats demonstrates the proof of concept that transdermal delivery can be an alternative route for a sustained, once-weekly systemic delivery of TAF.
Puri A, Bhattaccharjee SA, Zhang W, Clark M, Singh O, Doncel GF, Banga AK. Development of a Transdermal Delivery System for Tenofovir Alafenamide, a Prodrug of Tenofovir with Potent Antiviral Activity Against HIV and HBV. Pharmaceutics. 2019 Apr 9;11(4):173. doi: https://doi.org/10.3390/pharmaceutics11040173. PMID: 30970630; PMCID: PMC6523937.
Tenofovir alafenamide (TAF) is an effective nucleotide reverse transcriptase inhibitor that is used in the treatment of HIV-1 and HBV. Currently, it is being investigated for HIV prophylaxis. Oral TAF regimens require daily intake, which hampers adherence and increases the possibility of viral resistance. Long-acting formulations would significantly reduce this problem. Therefore, the aim of this study was to develop a transdermal patch containing TAF and investigate its performance in vitro through human epidermis. Two types of TAF patches were manufactured. Transparent patches were prepared using acrylate adhesive (DURO-TAK 87-2516), and suspension patches were prepared using silicone (BIO-PSA 7-4301) and polyisobutylene (DURO-TAK 87-6908) adhesives. In vitro permeation studies were performed while using vertical Franz diffusion cells for seven days. An optimized silicone-based patch was characterized for its adhesive properties and tested for skin irritation. The acrylate-based patches, comprising 2% w/w TAF and a combination of chemical enhancers, showed a maximum flux of 0.60 ± 0.09 µg/cm²/h. However, the silicone-based patch comprising of 15% w/w TAF showed the highest permeation (7.24 ± 0.47 μg/cm²/h). This study demonstrates the feasibility of developing silicone-based transdermal patches that can deliver a therapeutically relevant dose of TAF for the control of HIV and HBV infections.
Gunawardana M, Remedios-Chan M, Sanchez D, Webster S, Galvan P, Fanter R, Castonguay AE, Webster P, Moss JA, Kuo J, Gallay PA, Vincent KL, Motamedi M, Weinberger D, Marzinke MA, Hendrix CW, Baum MM. Multispecies Evaluation of a Long-Acting Tenofovir Alafenamide Subdermal Implant for HIV Prophylaxis. Front Pharmacol. 2020 Nov 25;11:569373. doi: https://doi.org/10.3389%2Ffphar.2020.569373. PMID: 33536904; PMCID: PMC7849190.
New HIV-1 infection rates far outpace the targets set by global health organizations, despite important progress in curbing the progression of the epidemic. Long-acting (LA) formulations delivering antiretroviral (ARV) agents for HIV-1 pre-exposure prophylaxis (PrEP) hold significant promise, potentially facilitating adherence due to reduced dosing frequency compared to oral regimens. We have developed a subdermal implant delivering the potent ARV drug tenofovir alafenamide that could provide protection from HIV-1 infection for 6 months, or longer. Implants from the same lot were investigated in mice and sheep for local safety and pharmacokinetics (PKs). Ours is the first report using these animal models to evaluate subdermal implants for HIV-1 PrEP. The devices appeared safe, and the plasma PKs as well as the drug and metabolite concentrations in dermal tissue adjacent to the implants were studied and contrasted in two models spanning the extremes of the body weight spectrum. Drug and drug metabolite concentrations in dermal tissue are key in assessing local exposure and any toxicity related to the active agent. Based on our analysis, both animal models were shown to hold significant promise in LA product development.
Zane D, Roller S, Shelton J, Singh R, Jain R, Wang Y, Yang B, Felx M, Alessi T, Feldman PL. A 28-Day Toxicity Study of Tenofovir Alafenamide Hemifumarate by Subcutaneous Infusion in Rats and Dogs. Microbiol Spectr. 2021 Sep 3;9(1):e0033921. doi: https://doi.org/10.1128/spectrum.00339-21. Epub 2021 Jun 30. PMID: 34190595; PMCID: PMC8552772.
The toxicity of tenofovir alafenamide (TAF) hemifumarate (HF) was evaluated when administered by continuous subcutaneous (s.c.) infusion via an external infusion pump for 28 days to rats and dogs. The toxicokinetics of TAF and two metabolites, tenofovir (TFV) and tenofovir diphosphate (TFV-DP) were also evaluated. After administration of TAF HF in rats and dogs, primary systemic findings supported an inflammatory response that was considered minimal to mild. Gross pathology and histopathologic evaluation of tissue surrounding the s.c. infusion site revealed signs of inflammation, including edema, mass formation, fibrosis, and mononuclear cell inflammation in groups receiving ≥300 μg/kg/day in rats and ≥25 μg/day in dogs. Although these changes were observed in animals receiving vehicle, the severity was greater in animals receiving TAF HF. Changes in the local tissue were considered a TAF HF-mediated exacerbation of an inflammatory response to the presence of the catheter. In rats, systemic and local findings were considered not adverse due to their low severity and reversibility; therefore, the "no observed adverse effect level" (NOAEL) was set at 1,000 μg/kg/day. Because none of the systemic findings were related to systemic exposure to TAF, the systemic NOAEL was set at 250 μg/kg/day in dogs. Due to the severity of the observations noted, a NOAEL for local toxicity could not be established. Although these results might allow for exploration of tolerability and pharmacokinetics of s.c. administered TAF HF in humans, data suggest a local reaction may develop in humans at doses below a clinically relevant dose. IMPORTANCE Human immunodeficiency virus (HIV) infection continues to be a serious global human health issue, with ∼38 million people living with HIV worldwide at the end of 2019. HIV preexposure prophylaxis (PrEP) has introduced the use of antiretroviral therapies as another helpful tool for slowing the spread of HIV worldwide. One possible solution to the problem of inconsistent access and poor adherence to HIV PrEP therapies is the development of subcutaneous (s.c.) depots or s.c. implantable devices that continuously administer protective levels of an HIV PrEP therapy for weeks, months, or even years at a time. We evaluate here the toxicity of tenofovir alafenamide, a potent inhibitor or HIV replication, after continuous s.c. infusion in rats and dogs for HIV PrEP.
Additional documents
No documents were uploaded
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Not provided