
|
Developed by 

|
Supported by 

|
Tenofovir-Lamivudine-Dolutegravir (TLD) - long-acting injectable (LAI) (TLD LAI)
Developer(s)

|
University of Washington https://www.washington.edu/United States The University of Washington is a public research university based in Seattle, Washington, USA. Originally founded in 1861, the institution has an extraordinary track record of scientific inventions & discoveries. Its Targeted Long-acting Combination Antiretroviral Therapy (TLC-ART) program aims to develop safe, stable, scalable and tolerable long-acting ART combinations for the treatment of HIV. |
Drug structure

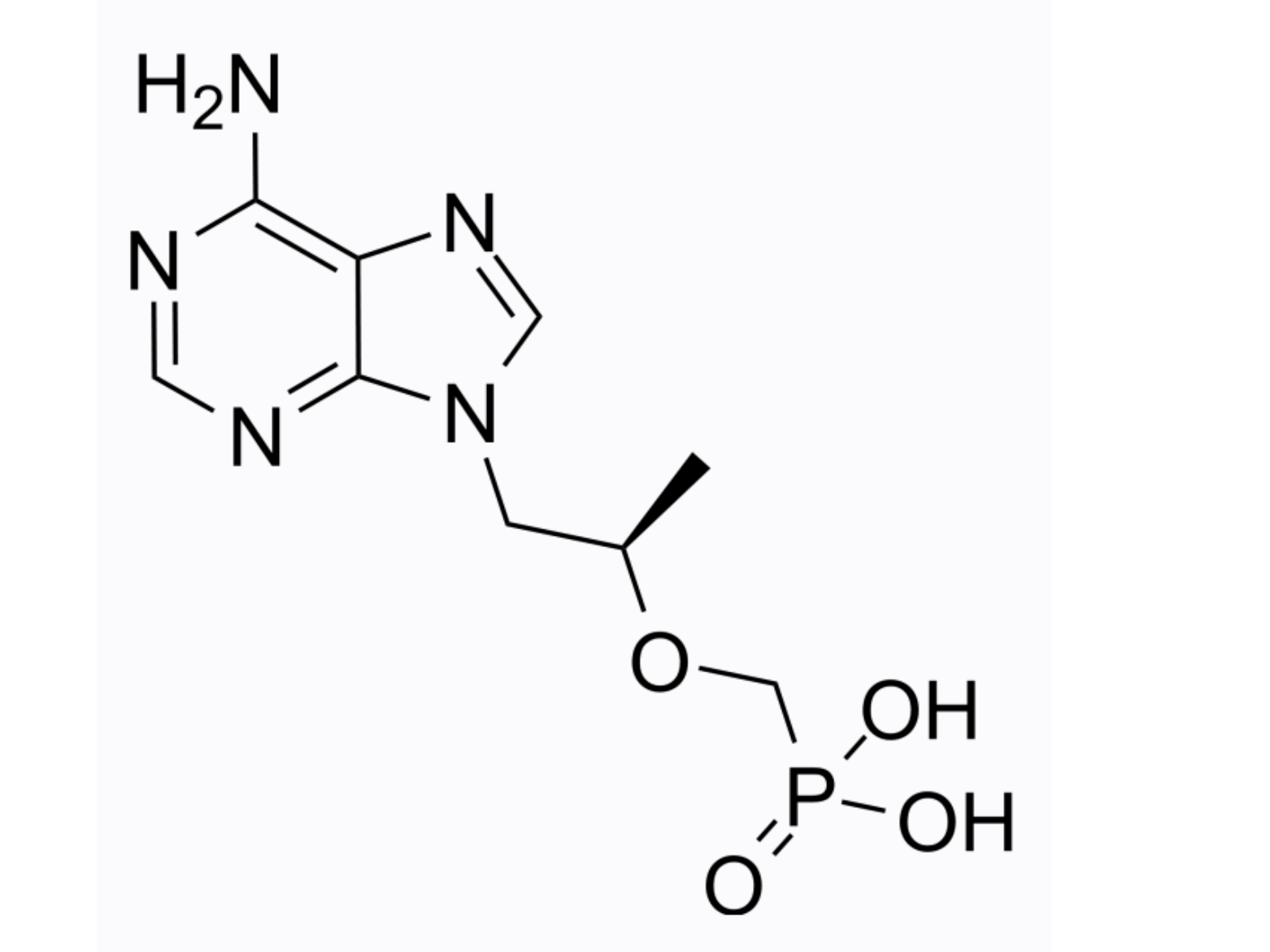
tenofovir (aka GS 1278 aka PMPA)
MedChemExpress
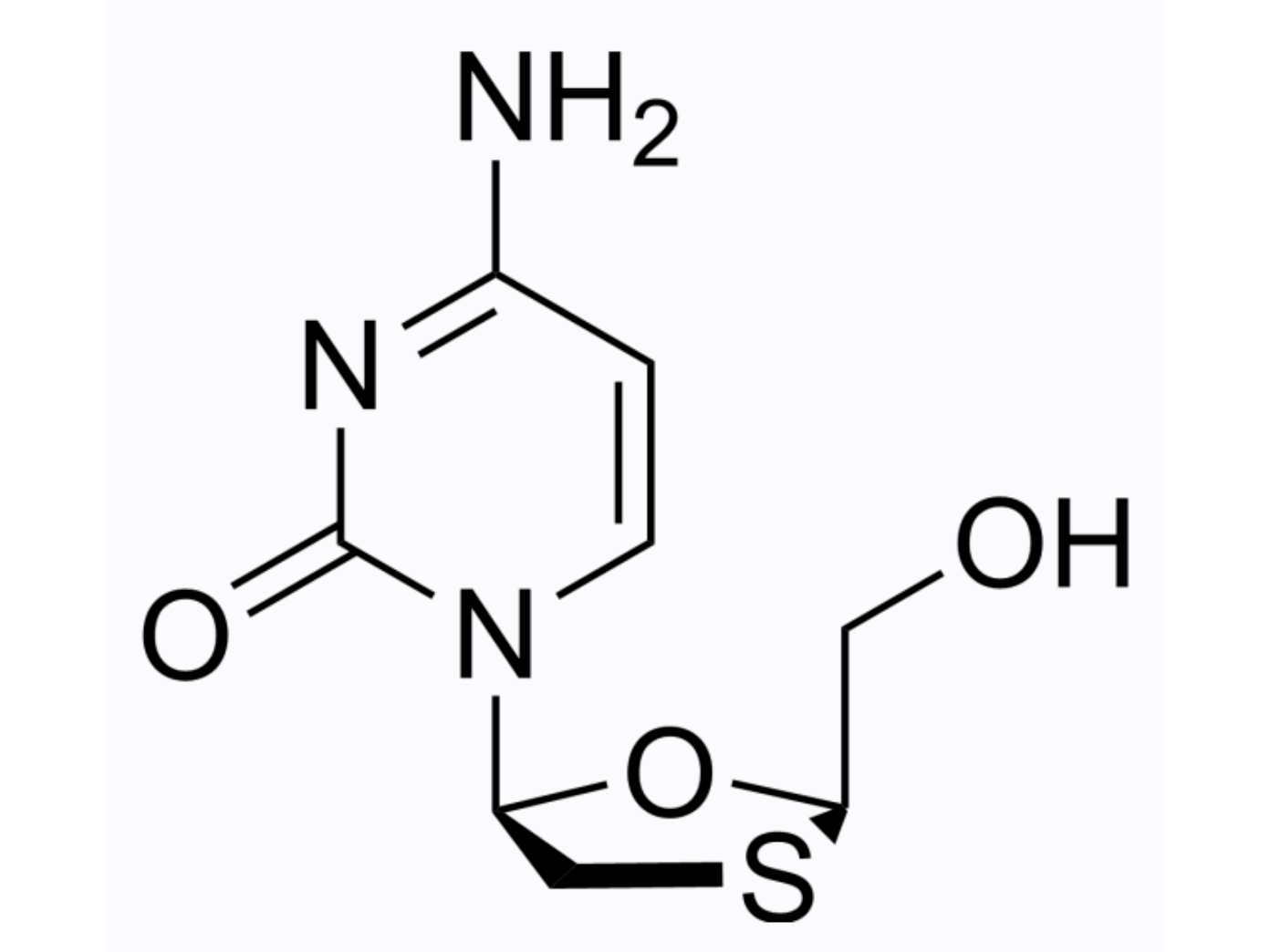
lamivudine (aka BCH-189)
MedChemExpress
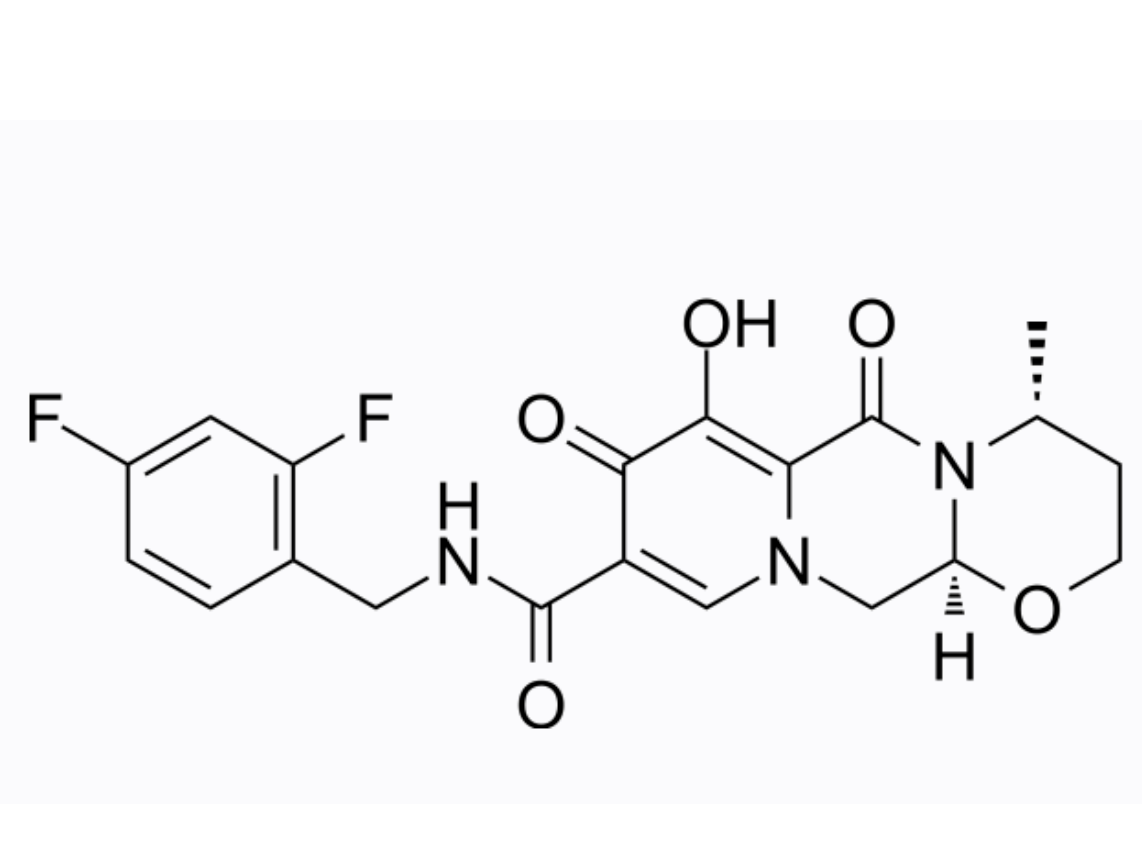
dolutegravir (aka S/GSK1349572)
MedChemExpress
Drug information
Associated long-acting platforms
Aqueous drug particle suspension, Based on other organic particles
Administration route
Subcutaneous
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
User acceptance
Not provided
Dosage
Available dose and strength
Not provided
Frequency of administration
Not provided
Maximum dose
Not provided
Recommended dosing regimen
Not provided
Additional comments
Not provided
Dosage link(s)
Not provided
Drug information
Drug's link(s)
Not provided
Generic name
Brand name
Compound type
Summary
Approval status
Regulatory authorities
Delivery device(s)
No delivery device
Scale-up and manufacturing prospects
Scale-up prospects
A novel long-acting TLD drug-combination nano-particulate (DcNP) formulation for subcutaneous injection was prepared with biocompatible lipid excipients. The highly-scalable DcNP technology enables drugs with disparate physiochemical properties to be formulated into products that remain stable in aqueous suspension. First, TLD was dissolved with lipid-excipients in hydrated-alcohol, followed by a controlled solvent-removal process to create the TLD-DcNP powder. Next, the TLD-DcNP particle-size was reduced (60-80 nm) resulting in a stable-injectable TLD product suitable for subcutaneous dosing.
Tentative equipment list for manufacturing
Rotary evaporator (rotavap). High pressure homogeniser (e.g. Emulsiflex-c5; Avestin Inc., Canada). Spray-dryer (e.g. 4M8Trix Unit; ProCepT, Belgium).
Manufacturing
TLD-in-DcNP injectable suspension was prepared by dissolving 40.27 mmol DSPC, 5.97mmol HCl, 5.66 mmol DTG and 4.49mmol mPEG2000-DSPE in 472 ml ethanol at 70°C. Following dissolution, 28 ml of 200 mM NaHCO3 buffer containing 5.85 mmol TFV and 5.85 mmol 3TC was added. The solution was then spray-dried under controlled-solvent-removal process to generate the TLD-in-DcNP powder. The powder in 0.45% w/v NaCl–20 mM NaHCO3 buffer suspension was held at 75°C and homogenised to achieve stable particles (50–70 nm). The suspension was cooled to 25°C and stored at 4°C.
Specific analytical instrument required for characterization of formulation
Particle size determined by photon correlation spectroscopy using a NICOMP 380 ZLS (Particle Sizing Systems, Santa Barbara, CA). Osmolality (Vapro 5520 osmometer; Wescor, Logan, UT) and pH (Hydrion paper). Drug quantification via LC-MS/MS using acetonitrile precipitation.
Clinical trials
Not providedExcipients
Proprietary excipients used
Lipid excipients: DSPC and DSPE-mPEG2000
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
Not provided
Residual solvents used
Not provided
Patent info
Compound patent families
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
TAF manufacturing process
Expiry date: 2032-10-03 Methods for isolating 9-{(R)-2-[((S)-{[(S)-l - (isopropoxycarbonyl)ethyl]amino}phenoxyphosphinyl)methoxy]propyl}adenine:'' (compound 16): a method for preparing, in high diastereomeric purity, intermediate compounds 13 and 15: method for preparing intermediate compound 12: 9-{(R)-2-[((S)-{[(S)-l - (isopropoxycarbonyl)ethyl]amino}phenoxyphosphinyl)methoxy]propyl}adenine has anti-viral properties. |
WO2013052094 | Process | Gilead Sciences, Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | China, Colombia, Mexico, Armenia, Azerbaijan, Belarus, Kyrgyzstan, Kazakhstan, Tajikistan, Turkmenistan, Türkiye, Bosnia and Herzegovina, Montenegro | Australia, Canada, Hong Kong, Japan, Korea, Republic of, Taiwan, Province of China, United States of America, Russian Federation, Austria, Belgium, Switzerland, Czechia, Germany, Spain, France, United Kingdom, Greece, Hungary, Ireland, Italy, Liechtenstein, Netherlands, Norway, Poland, Portugal, Sweden, Slovenia, Slovakia, New Zealand, Israel |
| Filed | China | Hong Kong, Korea, Republic of |
| Not in force | Argentina, Costa Rica, Peru, Albania, North Macedonia, Serbia, Türkiye, World Intellectual Property Organization (WIPO), Brazil, Bosnia and Herzegovina, Montenegro, Ecuador | Chile, Japan, Uruguay, Bulgaria, Cyprus, Czechia, Denmark, Estonia, Finland, Greece, Croatia, Hungary, Iceland, Lithuania, Luxembourg, Latvia, Monaco, Malta, Norway, Poland, Romania, Slovenia, Slovakia, San Marino, World Intellectual Property Organization (WIPO) |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on tenofovir disoproxil fumarate (TDF)
https://medicinespatentpool.org/licence-post/tenofovir-disoproxil-fumarate-tdf/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Compulsory licence on dolutegravir in Colombia
https://www.statnews.com/wp-content/uploads/2024/04/NC_534_Licencia_obligatoria_aceptada.pdfPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Tenofovir alafenamide hemifumarate (TAF)
Expiry date: 2032-08-15 A hemifumarate form of tenofovir alafenamide, and antiviral therapy using tenofovir alafenamide hemifurnarate (e.g., anti-HTV and anti-HBV therapies). |
WO2013025788 | Salt | Gilead Sciences, Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Costa Rica, Morocco, Moldova, Republic of, Mexico, Peru, Botswana, Ghana, Gambia (the), Kenya, Liberia, Lesotho, Malawi, Mozambique, Namibia, Rwanda, Sudan, Sierra Leone, Eswatini, Tanzania, United Republic of, Uganda, Zambia, Zimbabwe, Armenia, Azerbaijan, Belarus, Kyrgyzstan, Kazakhstan, Tajikistan, Turkmenistan, Viet Nam, Benin, Cameroon, Burkina Faso, Chad, Guinea-Bissau, Mali, Senegal, Congo, Guinea, Gabon, Niger, Equatorial Guinea, Mauritania, Togo, Côte d'Ivoire, Central African Republic, Bolivia (Plurinational State of), Philippines, South Africa, Ukraine, Brazil, El Salvador, Montenegro, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Serbia | United States of America, Australia, Canada, Chile, Hong Kong, Israel, Japan, Korea, Republic of, New Zealand, Singapore, Taiwan, Province of China, Uruguay, Russian Federation, Denmark, Slovenia, Panama, Croatia, San Marino, Cyprus, Bahamas, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Monaco, Portugal, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, Romania, Latvia, Lithuania |
| Filed | China, Ecuador, India, Paraguay, Thailand, Venezuela (Bolivarian Republic of), Türkiye, North Macedonia, Albania, Serbia, Egypt | Hong Kong, Denmark, Slovenia, Bahrain, Kuwait, Qatar, Saudi Arabia, Oman, United Arab Emirates, Croatia, San Marino, Cyprus, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Monaco, Portugal, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, Romania, Latvia, Lithuania |
| Not in force | World Intellectual Property Organization (WIPO), Argentina, China, Colombia, Indonesia, Pakistan, Brazil, Montenegro, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Serbia | World Intellectual Property Organization (WIPO), Hong Kong, Israel, Japan, New Zealand, Denmark, Slovenia, Croatia, San Marino, Cyprus, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Monaco, Portugal, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, Romania, Latvia, Lithuania |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on tenofovir disoproxil fumarate (TDF)
https://medicinespatentpool.org/licence-post/tenofovir-disoproxil-fumarate-tdf/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Compulsory licence on dolutegravir in Colombia
https://www.statnews.com/wp-content/uploads/2024/04/NC_534_Licencia_obligatoria_aceptada.pdfPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Dolutegravir in combination with lamivudine (3TC)
Expiry date: 2031-01-24 The present disclosure relates to combinations of compounds comprising HIV integrase inhibitors and other therapeutic agents. Such combinations may be useful in the inhibition of HIV-1 or potentially the inhibition of HIV replication, or for the prevention and/or treatment of infection by HIV, or in the treatment of AIDS and/or ARC. |
CA3003988 | Combination | Viiv Healthcare Company | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro, Serbia, Mexico | United States of America, Canada, Australia, Russian Federation, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania, Slovenia, Israel |
| Filed | Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro, Serbia | Singapore, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania, Slovenia |
| Not in force | Moldova, Republic of, Dominican Republic | United States of America, Australia |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on tenofovir disoproxil fumarate (TDF)
https://medicinespatentpool.org/licence-post/tenofovir-disoproxil-fumarate-tdf/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Compulsory licence on dolutegravir in Colombia
https://www.statnews.com/wp-content/uploads/2024/04/NC_534_Licencia_obligatoria_aceptada.pdfPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Dolutegravir or cabotegravir in combination with ABC, 3TC or RPV
Expiry date: 2031-01-24 The present invention relates to combinations of compounds comprising HIV integrase inhibitors and other therapeutic agents. Such combinations are useful in the inhibition of HIV replication, the prevention and/or treatment of infection by HIV, and in the treatment of AIDS and/or ARC. |
WO2011094150 | Combination | Glaxosmithkline Llc, Underwood, Mark Richard | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Malaysia, Philippines | Hong Kong |
| Filed | Algeria, Egypt, Thailand, Malaysia, Philippines, Viet Nam | Oman |
| Not in force | Costa Rica, Ecuador, Libya, World Intellectual Property Organization (WIPO), Brazil, Tajikistan, Belarus, Azerbaijan, Moldova, Republic of, Turkmenistan, Armenia, Kyrgyzstan, Kazakhstan | Hong Kong, World Intellectual Property Organization (WIPO), Russian Federation |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on tenofovir disoproxil fumarate (TDF)
https://medicinespatentpool.org/licence-post/tenofovir-disoproxil-fumarate-tdf/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Compulsory licence on dolutegravir in Colombia
https://www.statnews.com/wp-content/uploads/2024/04/NC_534_Licencia_obligatoria_aceptada.pdfPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Dolutegravir/Cabotegravir intermediates production processes & Intermediates
Expiry date: 2029-12-09 Processes are provided which create an aldehyde methylene, or hydrated or hemiacetal methylene attached to a heteroatom of a 6 membered ring without going through an olefinic group and without the necessity of using an osmium reagent. In particular, a compound of formula (I) can be produced from (II) and avoid the use of an allyl amine: (formulae I and II) where R, P 1 P3, R3 and Rx are as described herein. |
WO2010068262 | Intermediate(s), Process | Sionogi & Co., Ltd, Viiv Healthcare Company | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | China, India, North Macedonia | Japan, Korea, Republic of, Singapore, Taiwan, Province of China, United States of America, Portugal, Belgium, Germany, France, Netherlands, Switzerland, United Kingdom, Italy, Liechtenstein, Spain, Finland, Cyprus, Hungary |
| Filed | Portugal, Spain | |
| Not in force | China, World Intellectual Property Organization (WIPO), Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Serbia | World Intellectual Property Organization (WIPO), Luxembourg, Sweden, Austria, Greece, Denmark, Monaco, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania, Slovenia |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on tenofovir disoproxil fumarate (TDF)
https://medicinespatentpool.org/licence-post/tenofovir-disoproxil-fumarate-tdf/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Compulsory licence on dolutegravir in Colombia
https://www.statnews.com/wp-content/uploads/2024/04/NC_534_Licencia_obligatoria_aceptada.pdfPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Dolutegravir salts, their crystals & process
Expiry date: 2029-12-08 A synthesis approach providing an early ring attachment via a bromination to compound l-l yielding compound II-Il, whereby a final product such as AA can be synthesized. In particular, the 2,4-difluorophenyl-containing sidechain is attached before creation of the additional ring Q. |
WO2010068253 | Process, Salt | Glaxosmithkline Llc, Johns, Brian, Alvin, Shionogi & Co., Ltd, Taoda, Yoshiyuki, Yoshida, Hiroshi | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | China, Mexico, Brazil, Indonesia, India | United States of America, Australia, Canada, Switzerland, Germany, Spain, France, United Kingdom, Ireland, Italy, Liechtenstein, Japan, Korea, Republic of, Russian Federation, Singapore, Taiwan, Province of China |
| Filed | Canada | |
| Not in force | World Intellectual Property Organization (WIPO), North Macedonia, Türkiye, India | World Intellectual Property Organization (WIPO), Austria, Belgium, Bulgaria, Switzerland, Cyprus, Czechia, Germany, Denmark, Estonia, Spain, Finland, France, United Kingdom, Greece, Croatia, Hungary, Ireland, Iceland, Italy, Liechtenstein, Lithuania, Luxembourg, Latvia, Monaco, Malta, Netherlands, Norway, Poland, Portugal, Romania, Sweden, Slovenia, Slovakia, San Marino, Hong Kong |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on tenofovir disoproxil fumarate (TDF)
https://medicinespatentpool.org/licence-post/tenofovir-disoproxil-fumarate-tdf/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Compulsory licence on dolutegravir in Colombia
https://www.statnews.com/wp-content/uploads/2024/04/NC_534_Licencia_obligatoria_aceptada.pdfPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Cabotegravir prodrugs & Cabotegravir and Dolutegravir intermediates and processes
Expiry date: 2029-07-23 The present invention features compounds that are prodrugs of HIV integrase inhibitors and therefore are useful in the delivery of compounds for the inhibition of HIV replication, the prevention and/or treatment of infection by HIV, and in the treatment of AIDS and/or ARC. |
WO2010011814 | Intermediate(s), Process | Glaxosmithkline Llc, Shionogi & Co., Ltd, Viiv Healthcare Company | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | China, India | Belgium, Germany, France, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Liechtenstein, Spain, Portugal, Japan, Korea, Republic of, Singapore, United States of America |
| Filed | Spain, Portugal | |
| Not in force | Türkiye, North Macedonia, Bosnia and Herzegovina, World Intellectual Property Organization (WIPO), Albania, Serbia | Belgium, France, Luxembourg, Netherlands, Switzerland, Sweden, Austria, Liechtenstein, Greece, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania, Slovenia, World Intellectual Property Organization (WIPO) |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on tenofovir disoproxil fumarate (TDF)
https://medicinespatentpool.org/licence-post/tenofovir-disoproxil-fumarate-tdf/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Compulsory licence on dolutegravir in Colombia
https://www.statnews.com/wp-content/uploads/2024/04/NC_534_Licencia_obligatoria_aceptada.pdfPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Dolutegravir and Cabotegravir compounds
Expiry date: 2026-04-28 The present invention is to provide a novel compound (I), having the anti-virus activity, particularly the HIV integrase inhibitory activity, and a drug containing the same, particularly an anti-HIV drug, as well as a process and an intermediate thereof. Compound (I) wherein Z<1> is NR<4>; R<1> is hydrogen or lower alkyl; X is a single bond, a hetero atom group selected from O, S, SO, SO2 and NH, or lower alkylene or lower alkenylene in which the hetero atom group may intervene; R<2> is optionally substituted aryl; R<3> is hydrogen, a halogen, hydroxy, optionally substituted lower alkyl etc; and R<4> and Z<2> part taken together forms a ring, to form a polycyclic compound, including e.g., a tricyclic or tetracyclic compound. |
WO2006116764 | Compound | Glaxosmithkline Llc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Brazil, China, Morocco, Mexico, Philippines, Ukraine, Viet Nam, South Africa, Türkiye, Armenia, Azerbaijan, Belarus, Kyrgyzstan, Kazakhstan, Moldova, Republic of, Tajikistan, Turkmenistan, Nigeria, Colombia, Indonesia, Malaysia, Algeria | United States of America, Australia, Canada, Cyprus, Hong Kong, Israel, Japan, Korea, Republic of, Luxembourg, Norway, New Zealand, Taiwan, Province of China, Austria, Belgium, Bulgaria, Switzerland, Czechia, Germany, Denmark, Estonia, Spain, Finland, France, United Kingdom, Greece, Hungary, Ireland, Iceland, Italy, Liechtenstein, Lithuania, Latvia, Monaco, Netherlands, Poland, Portugal, Romania, Sweden, Slovenia, Slovakia, Russian Federation, Trinidad and Tobago, Singapore |
| Filed | Egypt | United States of America, Cyprus, Luxembourg, Norway, Finland, France, Hungary, Lithuania, Netherlands, Slovenia |
| Not in force | Türkiye, India, World Intellectual Property Organization (WIPO) | United States of America, Cyprus, Hong Kong, Israel, Japan, Luxembourg, Austria, Belgium, Bulgaria, Switzerland, Czechia, Germany, Denmark, Estonia, Spain, Finland, France, United Kingdom, Greece, Hungary, Ireland, Iceland, Italy, Liechtenstein, Lithuania, Latvia, Monaco, Netherlands, Poland, Portugal, Romania, Sweden, Slovenia, Slovakia, World Intellectual Property Organization (WIPO) |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on tenofovir disoproxil fumarate (TDF)
https://medicinespatentpool.org/licence-post/tenofovir-disoproxil-fumarate-tdf/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Compulsory licence on dolutegravir in Colombia
https://www.statnews.com/wp-content/uploads/2024/04/NC_534_Licencia_obligatoria_aceptada.pdfPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Tenofovir alafenamide fumarate (TAF)
Expiry date: 2021-07-20 A novel method is provided for screening prodrugs of methoxyphosphonate nucleotide analogues to identify prodrugs selectively targeting desired tissues with antiviral or antitumor activity. This method has led to the identification of novel mixed ester-amidates of PMPA for retroviral or hepadnaviral therapy, including compounds of structure (5a) having substituent groups as defined herein. Compositions of these novel compounds in pharmaceutically acceptable excipients and their use in therapy and prophylaxis are provided. Also provided is an improved method for the use of magnesium alkoxide for the preparation of starting materials and compounds for use herein. |
WO0208241 | Compound | Gilead Sciences, Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Ukraine, Albania, Ethiopia, Fiji, Grenada, Kiribati, Solomon Islands, Saint Lucia | Australia, Bulgaria, Denmark, Estonia, Hong Kong, Croatia, Hungary, Israel, Iceland, Japan, Korea, Republic of, Poland, Slovenia, United States of America, Russian Federation, Belgium, Switzerland, Cyprus, Germany, Finland, France, United Kingdom, Greece, Italy, Liechtenstein, Luxembourg, Netherlands, Sweden, Lithuania, Romania, Latvia, Brunei Darussalam, Czechia, Anguilla, Bermuda, Falkland Islands (Malvinas), Montserrat, Turks and Caicos Islands, Virgin Islands (British), Saint Helena, Ascension and Tristan da Cunha, Singapore, Cayman Islands, Gibraltar, Guernsey |
| Filed | Jamaica | Australia, Denmark, Spain, Norway, Portugal, Slovenia, Cyprus, Finland, France, Lithuania |
| Not in force | China, Mexico, Türkiye, South Africa, Ghana, Gambia (the), Kenya, Lesotho, Malawi, Mozambique, Sudan, Sierra Leone, Eswatini, Tanzania, United Republic of, Uganda, Zimbabwe, Armenia, Azerbaijan, Belarus, Kyrgyzstan, Kazakhstan, Moldova, Republic of, Tajikistan, Turkmenistan, Burkina Faso, Benin, Central African Republic, Congo, Côte d'Ivoire, Cameroon, Gabon, Guinea, Equatorial Guinea, Guinea-Bissau, Mali, Mauritania, Niger, Senegal, Chad, Togo, India, Indonesia, Viet Nam, World Intellectual Property Organization (WIPO), North Macedonia, Albania, Congo, democratic Republic of the, Haiti, Nepal, Tuvalu, Brazil | Canada, Australia, Denmark, Spain, Hong Kong, Croatia, Japan, Norway, New Zealand, Portugal, Slovenia, United States of America, Austria, Belgium, Switzerland, Cyprus, Germany, Finland, France, United Kingdom, Greece, Ireland, Italy, Liechtenstein, Luxembourg, Monaco, Netherlands, Sweden, World Intellectual Property Organization (WIPO), Lithuania, Romania, Latvia, Czechia, Guyana, Seychelles, Jersey |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on tenofovir disoproxil fumarate (TDF)
https://medicinespatentpool.org/licence-post/tenofovir-disoproxil-fumarate-tdf/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Compulsory licence on dolutegravir in Colombia
https://www.statnews.com/wp-content/uploads/2024/04/NC_534_Licencia_obligatoria_aceptada.pdfPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Tenofovir disoproxil fumarate (TDF)
Expiry date: 2018-07-23 The invention provides a composition comprising bis(POC)PMPA and fumaric acid (1:1). The composition is useful as an intermediate for the preparation of antiviral compounds, or is useful for administration to patients for antiviral therapy or prophylaxis. The composition is particularly useful when administered orally. The invention also provides methods to make PMPA and intermediates in PMPA synthesis. Embodiments include lithium t-butoxide, 9-(2-hydroxypropyl) adenine and diethyl p-toluenesulfonylmethoxy-phosphonate in an organic solvent such as DMF. The reaction results in diethyl PMPA preparations containing an improved by-product profile compared to diethyl PMPA made by prior methods |
WO9905150 | Compound, Salt | Gilead Sciences, Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | ||
| Filed | Portugal | |
| Not in force | Brazil, China, India, Mexico, Indonesia, World Intellectual Property Organization (WIPO), Albania | Canada, United States of America, Austria, Australia, Germany, Denmark, Spain, Hong Kong, Japan, Korea, Republic of, New Zealand, Portugal, Singapore, Slovenia, Taiwan, Province of China, Belgium, Switzerland, Cyprus, Finland, France, United Kingdom, Greece, Ireland, Italy, Liechtenstein, Luxembourg, Monaco, Netherlands, Sweden, World Intellectual Property Organization (WIPO), Lithuania, Latvia, Romania |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on tenofovir disoproxil fumarate (TDF)
https://medicinespatentpool.org/licence-post/tenofovir-disoproxil-fumarate-tdf/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Compulsory licence on dolutegravir in Colombia
https://www.statnews.com/wp-content/uploads/2024/04/NC_534_Licencia_obligatoria_aceptada.pdfPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Tenofovir disoproxil compounds
Expiry date: 2017-07-25 The present invention relates to intermediates for phosphonomethoxy nucleotide analogs, in particular intermediates suitable for use in the efficient oral delivery of such analogs. |
WO9804569 | Compound | Gilead Sciences, Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Luxembourg, United Kingdom | |
| Filed | ||
| Not in force | China, India, World Intellectual Property Organization (WIPO) | Canada, Austria, Australia, Germany, Denmark, Spain, Hong Kong, Japan, Korea, Republic of, Luxembourg, Netherlands, New Zealand, Portugal, Taiwan, Province of China, United States of America, Belgium, Switzerland, Finland, France, Greece, Ireland, Italy, Liechtenstein, Monaco, Sweden, Chile, World Intellectual Property Organization (WIPO), Singapore |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on tenofovir disoproxil fumarate (TDF)
https://medicinespatentpool.org/licence-post/tenofovir-disoproxil-fumarate-tdf/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Compulsory licence on dolutegravir in Colombia
https://www.statnews.com/wp-content/uploads/2024/04/NC_534_Licencia_obligatoria_aceptada.pdfPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Emtricitabine and lamivudine - process for preparing
Expiry date: 2012-12-21 The present invention relates to processes for preparing substituted 1,3-oxathiolanes with antiviral activity and intermediates of use in their preparation. |
WO9414802 | Process | Biochem Pharma Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | ||
| Filed | Denmark, Spain, Portugal | |
| Not in force | World Intellectual Property Organization (WIPO) | Canada, Austria, World Intellectual Property Organization (WIPO), Australia, Germany, Denmark, Spain, Hungary, Japan, Portugal, Belgium, Switzerland, France, United Kingdom, Greece, Ireland, Italy, Liechtenstein, Luxembourg, Monaco, Netherlands, Sweden |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on tenofovir disoproxil fumarate (TDF)
https://medicinespatentpool.org/licence-post/tenofovir-disoproxil-fumarate-tdf/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Compulsory licence on dolutegravir in Colombia
https://www.statnews.com/wp-content/uploads/2024/04/NC_534_Licencia_obligatoria_aceptada.pdfPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Lamivudine crystal forms
Expiry date: 2012-06-02 (-)$i(cis)-4-Amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(IH)-pyrimidine-2-one in crystalline form, in particular as needle-shaped or bipyramidyl crystals, pharmaceutical formulations thereof, methods for their preparation and their use in medicine. |
WO9221676 | Polymorphs | Glaxo Group Limited | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | United States of America | |
| Filed | United Kingdom, Austria, Australia, Denmark, Ireland, Iceland, Portugal, Singapore | |
| Not in force | Mexico, South Africa, Botswana, Ghana, Gambia (the), Kenya, Lesotho, Malawi, Sudan, Eswatini, Uganda, Zambia, Zimbabwe, Burkina Faso, Benin, Central African Republic, Congo, Côte d'Ivoire, Cameroon, Gabon, Guinea, Mali, Mauritania, Niger, Senegal, Chad, Togo, Philippines, Ukraine, Pakistan, Georgia, World Intellectual Property Organization (WIPO) | Canada, United Kingdom, Austria, Bulgaria, Germany, Denmark, Hong Kong, Ireland, Israel, Japan, Korea, Republic of, Norway, New Zealand, Portugal, Russian Federation, Slovakia, Taiwan, Province of China, Belgium, Switzerland, Spain, France, Greece, Italy, Liechtenstein, Luxembourg, Monaco, Netherlands, Sweden, World Intellectual Property Organization (WIPO) |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on tenofovir disoproxil fumarate (TDF)
https://medicinespatentpool.org/licence-post/tenofovir-disoproxil-fumarate-tdf/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Compulsory licence on dolutegravir in Colombia
https://www.statnews.com/wp-content/uploads/2024/04/NC_534_Licencia_obligatoria_aceptada.pdfPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Lamivudine compound
Expiry date: 2011-05-02 (-)-4-Amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one, its pharmaceutically acceptable derivatives, pharmaceutical formulations thereof, methods for its preparation and its use as an antiviral agent are described. |
WO9117159 | Compound | Iaf Biochem International Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | United States of America | |
| Filed | United Kingdom, Australia, Finland, Hungary, Japan, Korea, Republic of, New Zealand, Poland, Singapore, Slovenia | |
| Not in force | World Intellectual Property Organization (WIPO), Bosnia and Herzegovina, Yugoslavia/Serbia and Montenegro, China, Morocco, Moldova, Republic of, Tunisia, South Africa, Botswana, Ghana, Gambia (the), Kenya, Lesotho, Malawi, Sudan, Eswatini, Uganda, Zambia, Zimbabwe, Burkina Faso, Benin, Central African Republic, Congo, Côte d'Ivoire, Cameroon, Gabon, Guinea, Mali, Mauritania, Niger, Senegal, Chad, Togo, Egypt, Ukraine, Malaysia, Georgia | Canada, United Kingdom, World Intellectual Property Organization (WIPO), Bulgaria, Hong Kong, Croatia, Ireland, Israel, Japan, Korea, Republic of, Norway, Portugal, Romania, Slovakia, Taiwan, Province of China, United States of America, Austria, Belgium, Switzerland, Germany, Denmark, Spain, France, Greece, Italy, Liechtenstein, Luxembourg, Netherlands, Sweden |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on tenofovir disoproxil fumarate (TDF)
https://medicinespatentpool.org/licence-post/tenofovir-disoproxil-fumarate-tdf/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Compulsory licence on dolutegravir in Colombia
https://www.statnews.com/wp-content/uploads/2024/04/NC_534_Licencia_obligatoria_aceptada.pdfPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Emtricitabine and lamivudine compounds
Expiry date: 2010-02-08 Novel substituted 1,3-oxathiolane cyclic compounds having pharmacological activity, to processes for and intermediates of use in their preparation, to pharmaceutical compositions containing them, and to the use of these compounds in the antiviral treatment of mammals. |
CA2009637 | Compound | Biochem Pharma Inc, Iaf Biochem International, Inc | Yes |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | United States of America, Australia, Germany, Spain, Finland, Ireland, Portugal | |
| Filed | United States of America, Australia, Cyprus, Germany, Denmark, Spain, Greece, Hungary, Ireland, Japan, Luxembourg, Latvia, Netherlands, New Zealand, Poland, Singapore, Slovenia, Slovakia | |
| Not in force | Bosnia and Herzegovina, Yugoslavia/Serbia and Montenegro, China, Honduras, South Africa, Botswana, Ghana, Gambia (the), Kenya, Lesotho, Malawi, Sudan, Eswatini, Uganda, Zambia, Zimbabwe, Burkina Faso, Benin, Central African Republic, Congo, Côte d'Ivoire, Cameroon, Gabon, Guinea, Mali, Mauritania, Niger, Senegal, Chad, Togo, Malaysia, Philippines, Armenia, Kyrgyzstan, Tajikistan, Sri Lanka, Dominican Republic, Georgia, Uzbekistan, Mexico, Moldova, Republic of, Ukraine | Canada, United States of America, Austria, Germany, Denmark, Spain, Greece, Hong Kong, Croatia, Israel, Japan, Korea, Republic of, Luxembourg, Netherlands, Norway, Belgium, Switzerland, France, United Kingdom, Italy, Liechtenstein, Sweden, Uruguay, Saudi Arabia |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on tenofovir disoproxil fumarate (TDF)
https://medicinespatentpool.org/licence-post/tenofovir-disoproxil-fumarate-tdf/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Compulsory licence on dolutegravir in Colombia
https://www.statnews.com/wp-content/uploads/2024/04/NC_534_Licencia_obligatoria_aceptada.pdfSupporting material
Publications
Perazzolo S, Stephen ZR, Eguchi M, Xu X, Delle Fratte R, Collier AC, Melvin AJ, Ho RJY. A novel formulation enabled transformation of 3-HIV drugs tenofovir-lamivudine-dolutegravir from short-acting to long-acting all-in-one injectable. AIDS. 2023 Nov 15;37(14):2131-2136. DOI: 10.1097/QAD.0000000000003706. Epub 2023 Aug 24. PMID: 37650755; PMCID: PMC10959254.
Objective: To develop an injectable dosage form of the daily oral HIV drugs, tenofovir (T), lamivudine (L), and dolutegravir (D), creating a single, complete, all-in-one TLD 3-drug-combination that demonstrates long-acting pharmacokinetics.
Design: Using drug-combination-nanoparticle (DcNP) technology to stabilize multiple HIV drugs, the 3-HIV drugs TLD, with disparate physical-chemical properties, are stabilized and assembled with lipid-excipients to form TLD-in-DcNP . TLD-in-DcNP is verified to be stable and suitable for subcutaneous administration. To characterize the plasma time-courses and PBMC concentrations for all 3 drugs, single subcutaneous injections of TLD-in-DcNP were given to nonhuman primates (NHP, M. nemestrina ).
Results: Following single-dose TLD-in-DcNP , all drugs exhibited long-acting profiles in NHP plasma with levels that persisted for 4 weeks above predicted viral-effective concentrations for TLD in combination. Times-to-peak were within 24 hr in all NHP for all drugs. Compared to a free-soluble TLD, TLD-in-DcNP provided exposure enhancement and extended duration 7.0-, 2.1-, and 20-fold as AUC boost and 10-, 8.3-, and 5.9-fold as half-life extension. Additionally, DcNP may provide more drug exposure in cells than plasma with PBMC-to-plasma drug ratios exceeding one, suggesting cell-targeted drug-combination delivery.
Conclusions: This study confirms that TLD with disparate properties can be made stable by DcNP to enable TLD concentrations of 4 weeks in NHP. Study results highlighted the potential of TLD-in-DcNP as a convenient all-in-one, complete HIV long-acting product for clinical development.
Additional documents
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Not provided