
|
Developed by 

|
Supported by 

|
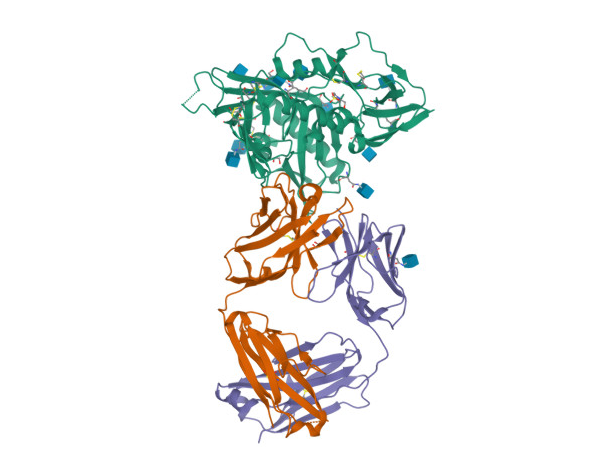
Teropavimab and Zinlirvimab
Developer(s)

|
Gilead Sciences Inc. https://www.gilead.com/United States Gilead Sciences, Inc. is a multinational biopharmaceutical company that develops and manufactures innovative medicines for life-threatening diseases, including anti-viral therapeutics for HIV/AIDS, Hepatitis B, Hepatitis C and Covid-19. Headquartered in Foster City, California, Gilead was originally founded in 1987 and is currently listed on both the S&P 500 and the NASDAQ Biotechnology Index. |
Drug structure
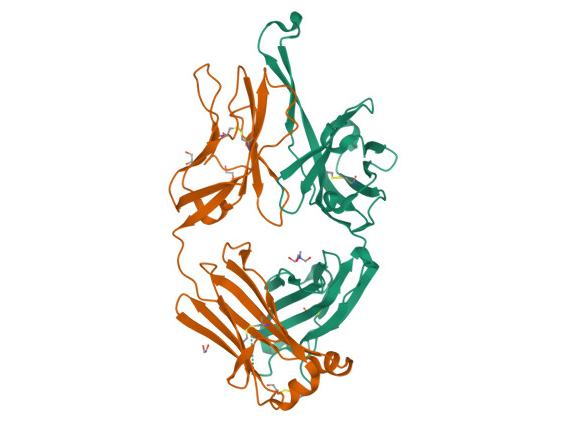
10-1074 Fragment Antigen Binding Region
http://doi.org/10.2210/pdb4FQ2/pdb

3BNC117 in Complex with HIV-1 Envelope Glycoprotein GP120
http://doi.org/10.2210/pdb4JPV/pdb
Drug information
Associated long-acting platforms
Broadly neutralising monoclonal antibody
Administration route
Intravenous, Subcutaneous
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
User acceptance
Not provided
Dosage
Available dose and strength
Not provided
Frequency of administration
Not provided
Maximum dose
Not provided
Recommended dosing regimen
Not provided
Additional comments
Not provided
Dosage link(s)
Not provided
Drug information
Drug's link(s)
Not provided
Generic name
Brand name
Compound type
Summary
Approval status
Regulatory authorities
Delivery device(s)
No delivery device
Scale-up and manufacturing prospects
Scale-up prospects
Production scale up and manufacturing requirements for therapeutic monoclonal antibody products are primarily related to formulation stability, pharmacokinetic suitability and maintenance of quality attributes. The industrial manufacture of high-concentration broadly neutralising antibody (bNAb) formulations for parenteral administration can introduce production challenges regarding aggregation propensity and formulation viscosity. Exploratory process optimisations such as bNAb co-formulation and multi-specific antibody composition have the potential to reduce overall manufacturing costs.
Tentative equipment list for manufacturing
Industrial bioreactor vessel with a production volume capacity of between 5-25kL. Continuous disc stack centrifuges for bioreactor harvesting with subsequent membrane and depth filtration for supernatant clarification. Protein A chromatography or other suitable affinity capture apparatus followed by two chromatographic polishing steps such as cation- and anion-exchange. Ultrafiltration membrane system to concentrate and formulate the final product.
Manufacturing
Biological activity of bNAbs is highly dependant on their chemical, conformational and structural stability. Reduced glycosylation of bNAbs during manufacture and chemical degradation processes such as deamidation can result in increased aggregation, loss of activity and diminished solubility. Degradation may occur at any stage throughout the manufacturing process including bioprocessing, purification, product delivery and storage. Considerations to increase formulation stability may include pH optimisation and the addition of suitable excipients (e.g. surfactants, stabilizers and buffers).
Specific analytical instrument required for characterization of formulation
Formulation characterisation for single-entity bNAb production include capillary isoelectric focusing and ion-exchange chromatography for identification of post-translational modifications, subvisible particle quantitation, thermal DSC, size-exclusion chromatography for measurement of concentration dependent aggregation rates and capillary electrophoresis for antibody fragmentation and clipping. Co-formulated bNAbs in mixture may utilise SE-HPLC, capillary electrophoresis sodium dodecyl sulphate, dynamic light scattering, microflow imaging and multi-attribute method mass spectrometry analysis.
Clinical trials
YCO-0946
Identifier
NCT03254277
Link
https://clinicaltrials.gov/study/NCT03254277
Phase
Phase I
Status
Completed
Sponsor
Rockefeller University
More details
Not provided
Purpose
Evaluate the safety, tolerability and pharmacokinetics of a single administration of 3BNC117-LS in HIV-uninfected and HIV-1 infected participants.
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2017-09-13
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2020-12-31
Actual Completion Date
2020-12-31
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Study participants placed into groups of HIV-infected and HIV-uninfected individuals. Inclusion criteria for HIV positive groups: Males and females aged 18-65 who have documented HIV-1 infection with CD4+ T cell counts of > 300 cells/μL and currently receiving antiretroviral therapy (ART) with < 20 copies/ml plasma HIV-1 RNA or not receiving ART for a minimum of 8 weeks with < 100,000 copies/ml plasma HIV-1 RNA. Inclusion criteria for HIV negative groups: Males and females aged 18-65 who have low risk for HIV infection and agree to implement two methods of effective contraception if sexually active.
Health status
Study type
Interventional (clinical trial)
Enrollment
43
Allocation
Non-randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (open label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
A5364
Identifier
NCT05079451
Link
https://clinicaltrials.gov/study/NCT05079451
Phase
Phase I
Status
Withdrawn
Sponsor
National Institute of Allergy and Infectious Diseases (NIAID)
More details
Protocol Withdrawal.
Purpose
Evaluate the ability and safety of the combined bNAbs 10-1074-LS and 3BNC117-LS to prevent viral rebound during an analytical antiretroviral treatment interruption.
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2024-01-01
Actual Start Date
Not provided
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
2024-01-15
Estimated Completion Date
2024-02-15
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Individuals aged 18-70 years with confirmed HIV-1 infection who have received a stable suppressive antiretroviral regimen (< 50 copies/ml plasma HIV-1 RNA levels) for a minimum of 48 weeks prior to enrolment with no reported continuous interruption of a treatment greater than 7 days. CD4+ T cell counts of > 450 cells/µL with a nadir of ≥200 cells µL is required for trial eligibility.
Health status
Study type
Interventional (clinical trial)
Enrollment
Not provided
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
RIO
Identifier
NCT04319367
Link
https://clinicaltrials.gov/study/NCT04319367
Phase
Phase II
Status
Recruiting
Sponsor
Imperial College London
More details
Not provided
Purpose
Evaluate whether the combination of 3BNC117-LS and 10-1074-LS can prevent HIV viral rebound after discontinuing early-initiation antiretroviral treatment in adults during primary HIV infection.
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-05-17
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
2027-07-31
Estimated Completion Date
2027-07-31
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Individuals aged 18-60 who are currently receiving a stable antiretroviral therapy (ART) regimen resulting in an undetectable HIV viral load for a time period of at least one year, which commenced within three months of documented primary HIV infection. Current CD+ T cell counts > 500 cells/µL with a nadir of CD4+ > 350 cells/µL are required. Study participants were required to be vaccinated against COVID-19 at least 28 days before enrolment.
Health status
Study type
Interventional (clinical trial)
Enrollment
72
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Triple (Participant, Investigator, Outcomes Assessor)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
RHIVIERA-02
Identifier
NCT05300035
Link
https://clinicaltrials.gov/study/NCT05300035
Phase
Phase II
Status
Recruiting
Sponsor
ANRS, Emerging Infectious Diseases
More details
Not provided
Purpose
Evaluate the efficacy of an intervention consisting of the long-acting broadly neutralising antibodies 3BNC117-LS and 10-1074-LS + ART in reducing HIV-1 replication during primary HIV-1 infection.
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2024-04-11
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
2026-12-10
Estimated Completion Date
2028-12-10
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Study participants are individuals with confirmed HIV-1 infection aged 18-70 at screening and no prior history of hypersensitivity or contraindication to 10-1074-LS or 3BNC117-LS intravenous infusions.
Health status
Study type
Interventional (clinical trial)
Enrollment
69
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Triple (Participant, Care Provider, Investigator)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
GS-US-536-5816
Identifier
NCT04811040
Link
https://clinicaltrials.gov/study/NCT04811040
Phase
Phase I
Status
Completed
Sponsor
Gilead Sciences
More details
Not provided
Purpose
Evaluate the safety and tolerability of a combination of the broadly neutralizing antibodies (bNAbs) teropavimab (formerly GS-5423) and GS-2872 in combination with the HIV capsid inhibitor Lenacapavir
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-04-08
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2023-04-18
Actual Completion Date
2023-10-17
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Study participants are required to have received an initial antiretroviral treatment regimen for two years or more prior to screening with no documented virological resistance. Individuals are permitted to change their ART regimen within 28 days prior to screening for reasons other than treatment resistance (e.g. drug-drug interactions, simplification, tolerability). Screening counts of CD4+ T cells ≥ 500 cells/μL with a nadir of ≥ 350 cells/μL are required, in addition to plasma HIV-1 RNA levels of < 50 copies/ml.
Health status
Study type
Interventional (clinical trial)
Enrollment
32
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Double-blind masking
Masking description
Double (Participant, Investigator)
Frequency of administration
Not provided
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
MCA-1031
Identifier
NCT05245292
Link
https://clinicaltrials.gov/study/NCT05245292
Phase
Phase I
Status
Recruiting
Sponsor
Rockefeller University
More details
Not provided
Purpose
Evaluate the antiretroviral activity and safety of the broadly neutralising antibodies 3BNC117-LS and 10-1074-LS in combination with IL-15 superagonist complex N-803.
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-12-07
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
2025-12-31
Estimated Completion Date
2025-12-31
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Male
- Female
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Study participants are males and females aged 18-70 with a confirmed HIV-1 infection who are currently receiving a stable antiretroviral treatment regimen (< 50 copies/ml plasma HIV-1 RNA) for at least 48 weeks with no reported continuous interruption of treatment greater than 7 days. CD4+ T cell counts were required to be > 450 cells/μL at enrolment with a cell count nadir of ≥ 200 cells/μL and HIV-1 RNA plasma levels at < 20 copies/ml.
Health status
Study type
Interventional (clinical trial)
Enrollment
36
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
MCA-0994
Identifier
NCT04250636
Link
https://clinicaltrials.gov/study/NCT04250636
Phase
Phase I
Status
Completed
Sponsor
Rockefeller University
More details
Not provided
Purpose
Evaluate the antiviral activity, pharmacokinetics and safety of single intravenous infusions of the bNAbs 3BNC117-LS and 10-1074-LS in HIV-infected individuals who are not currently receiving ART.
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2020-10-13
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-01-21
Actual Completion Date
2022-02-11
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Study participants are individuals with HIV-1 infection who have not received antiretroviral therapy (either by choice, intolerance or ART-naïvety) for at least 28 days prior to study enrolment with plasma HIV-1 RNA levels between 500 - 100,000 copies/mL and CD4+ T cell counts > 300 cells/μl.
Health status
Study type
Interventional (clinical trial)
Enrollment
6
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
YCO-0971
Identifier
NCT03554408
Link
https://clinicaltrials.gov/study/NCT03554408
Phase
Phase I
Status
Completed
Sponsor
Rockefeller University
More details
Not provided
Purpose
Evaluate the pharmacokinetic profile, tolerability and safety of 10-1074-LS in the first clinical study administered individually or in combination with 3BNC117-LS to individuals with and without HIV.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2018-06-20
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2021-02-04
Actual Completion Date
2021-02-04
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Study participants placed into groups of HIV-infected and HIV-uninfected individuals. Inclusion criteria for HIV positive groups: Males and females aged 18-65 who have documented HIV-1 infection and are currently receiving antiretroviral therapy with < 50 copies/ml plasma HIV-1 RNA levels and CD4+ T cell count of > 300 cells/μL. Inclusion criteria for HIV negative groups: Males and females aged 18-65 who have low risk for HIV infection and agree to implement two methods of effective contraception if sexually active.
Health status
Study type
Interventional (clinical trial)
Enrollment
77
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Double-blind masking
Masking description
Double (Participant, Investigator)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
NCT05612178
Identifier
NCT05612178
Link
https://clinicaltrials.gov/study/NCT05612178
Phase
Phase I
Status
Recruiting
Sponsor
National Institute of Allergy and Infectious Diseases (NIAID)
More details
Not provided
Purpose
Evaluate the safety and effects of repeated doses of 3BNC117-LS and 10-1074-LS on persistent viral reservoirs in people living with HIV who are currently receiving suppressive antiretroviral therapy.
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-07-26
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
2025-12-31
Estimated Completion Date
2025-12-31
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Adult persons of any sex or gender, aged 18 years to 70; with confirmed HIV-1 infection and receiving antiretroviral therapy with plasma HIV-1 RNA levels of < 50 copies/mL and no reported interruption of ART for 7 consecutive days or longer for at least 96 weeks.
Health status
Study type
Interventional (clinical trial)
Enrollment
200
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Triple (Participant, Care Provider, Investigator)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
PAUSE
Identifier
NCT06031272
Link
https://clinicaltrials.gov/study/NCT06031272
Phase
Phase I
Status
Not yet recruiting
Sponsor
AIDS Clinical Trials Group
More details
Not provided
Purpose
Evaluate the safety, antiviral activity, and immunomodulatory effects of co-administered 3BNC117-LS-J and 10-1074-LS-J in ART-treated adults in Sub-Saharan Africa.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2024-05-15
Actual Start Date
Not provided
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
2025-06-30
Estimated Completion Date
2026-06-30
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Participants are individuals aged 18 to 70 years with a confirmed HIV-1 infection and who have received stable suppressive ART for at least 96 weeks prior to study entry. Eligible participants must also display a CD4+ cell count of >450 cells/µL obtained within the previous 56 days and plasma HIV-1 RNA levels of <50 copies/mL for at least 96 weeks prior to study entry.
Health status
Study type
Interventional (clinical trial)
Enrollment
48
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Double-blind masking
Masking description
Double (Participant, Investigator)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
IAVI C100
Identifier
NCT04173819
Link
https://clinicaltrials.gov/study/NCT04173819
Phase
Phase I/II
Status
Active, not recruiting
Sponsor
International AIDS Vaccine Initiative
More details
Not provided
Purpose
Evaluate the safety and pharmacokinetics of the combination broadly neutralizing antibodies, 3BNC117-LS-J and 10-1074-LS-J, in healthy American and African Adults.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-01-25
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
2023-09-01
Estimated Completion Date
2023-09-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Healthy male and female individuals aged between 18-45 who are willing to undergo HIV testing, risk reduction counselling and receive HIV test results; in addition to maintaining low-risk behaviour for the entire trial duration.
Health status
Study type
Interventional (clinical trial)
Enrollment
225
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Double-blind masking
Masking description
Double (Participant, Investigator)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key resources
GS-US-536-5939
Identifier
NCT05729568
Link
https://clinicaltrials.gov/study/NCT05729568
Phase
Phase II
Status
Active, not recruiting
Sponsor
Gilead Sciences
More details
Not provided
Purpose
Evaluate the Safety and Efficacy of bNAbs GS-5423 and GS-2872 in Combination With Lenacapavir as Long-Acting Treatment Dosed Every 6 Months in Virologically Suppressed Adults With HIV-1 Infection.
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-05-15
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
2025-03-01
Estimated Completion Date
2029-12-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Participants are required to be receiving a stable ART regimen with no clinically significant documented resistance (except isolated NRTI mutations). Plasma HIV-1 RNA < 50 copies/mL at screening visit 2 and documented plasma HIV-1 RNA < 50 copies/mL for ≥ 12 months preceding screening visit 2.
Health status
Study type
Interventional (clinical trial)
Enrollment
83
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
None (Open Label)
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
Excipients
Proprietary excipients used
Not provided
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
Not provided
Residual solvents used
Not provided
Patent info
Description
Zinlirvimab+Teropamivimab combination
Brief description
Zinlirvimab variants + combination with Teropamivimab (Heavy chain e.g. SEQ 59-61)
Representative patent
WO2020056145
Category
Combination of active substances
Patent holder
The Rockefeller University
Exclusivity
Not provided
Expiration date
September 12, 2039
Status
Filed in China, India, Us, EP
Description
Zinlirvimab- Broadly neutralising anti-HIV antibodies
Brief description
The invention relates to anti-HIV antibodies. Also disclosed are related methods and compositions.
Representative patent
WO2014063059
Category
Active substance
Patent holder
The Rockefeller University; California Institute of Technology
Exclusivity
Not provided
Expiration date
October 18, 2033
Status
Granted in China, US, EAPO, EP
Description
Teropavimab - HIV neutralizing antibodies and methods of use thereof
Brief description
The invention provides broadly neutralizing antibodies directed to epitopes of Human Immunodeficiency Virus, or HIV. The invention further provides compositions containing HIV antibodies used for prophylaxis, and methods for diagnosis and treatment of HIV infection. Claims include antibody comprising aa 1-112 teropavimab H chain and 1-89 of L chain
Representative patent
WO2012158948
Category
Active substance
Patent holder
The Rockefeller University; California Institute of Technology
Exclusivity
Not provided
Expiration date
May 17, 2032
Status
Granted in US, EAPO, EP
Supporting material
Publications
Gautam, R., Nishimura, Y., Gaughan, N. et al. A single injection of crystallizable fragment domain–modified antibodies elicits durable protection from SHIV infection. Nat Med 24, 610–616 (2018). https://doi.org/10.1038/s41591-018-0001-2
In the absence of an effective and safe vaccine against HIV-1, the administration of broadly neutralizing antibodies (bNAbs) represents a logical alternative approach to prevent virus transmission. Here, we introduced two mutations encoding amino acid substitutions (M428L and N434S, collectively referred to as ‘LS’) into the genes encoding the crystallizable fragment domains of the highly potent HIV-specific 3BNC117 and 10-1074 bNAbs to increase their half-lives and evaluated their efficacy in blocking infection following repeated low-dose mucosal challenges of rhesus macaques (Macaca mulatta) with the tier 2 SHIVAD8-EO. A single intravenous infusion of 10-1074-LS monoclonal antibodies markedly delayed virus acquisition for 18 to 37 weeks (median, 27 weeks), whereas the protective effect of the 3BNC117-LS bNAb was more modest (provided protection for 11–23 weeks; median, 17 weeks). Serum concentrations of the 10-1074-LS monoclonal antibody gradually declined and became undetectable in all recipients between weeks 26 and 41, whereas the 3BNC117-LS bNAb exhibited a shorter half-life. To model immunoprophylaxis against genetically diverse and/or neutralization-resistant HIV-1 strains, a combination of the 3BNC117-LS plus 10-1074-LS monoclonal antibodies was injected into macaques via the more clinically relevant subcutaneous route. Even though the administered mixture contained an amount of each bNAb that was nearly threefold less than the quantity of the single monoclonal antibody in the intravenous injections, the monoclonal antibody combination still protected macaques for a median of 20 weeks. The extended period of protection observed in macaques for the 3BNC117-LS plus 10-1074-LS combination could translate into an effective semiannual or annual immunoprophylaxis regimen for preventing HIV-1 infections in humans.
Mendoza P, Gruell H, Nogueira L, Pai JA, Butler AL, Millard K, Lehmann C, Suárez I, Oliveira TY, Lorenzi JCC, Cohen YZ, Wyen C, Kümmerle T, Karagounis T, Lu CL, Handl L, Unson-O'Brien C, Patel R, Ruping C, Schlotz M, Witmer-Pack M, Shimeliovich I, Kremer G, Thomas E, Seaton KE, Horowitz J, West AP Jr, Bjorkman PJ, Tomaras GD, Gulick RM, Pfeifer N, Fätkenheuer G, Seaman MS, Klein F, Caskey M, Nussenzweig MC. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature. 2018 Sep;561(7724):479-484. DOI: 10.1038/s41586-018-0531-2. Epub 2018 Sep 26. PMID: 30258136; PMCID: PMC6166473.
Individuals infected with HIV-1 require lifelong antiretroviral therapy, because interruption of treatment leads to rapid rebound viraemia. Here we report on a phase 1b clinical trial in which a combination of 3BNC117 and 10-1074, two potent monoclonal anti-HIV-1 broadly neutralizing antibodies that target independent sites on the HIV-1 envelope spike, was administered during analytical treatment interruption. Participants received three infusions of 30 mg kg-1 of each antibody at 0, 3 and 6 weeks. Infusions of the two antibodies were generally well-tolerated. The nine enrolled individuals with antibody-sensitive latent viral reservoirs maintained suppression for between 15 and more than 30 weeks (median of 21 weeks), and none developed viruses that were resistant to both antibodies. We conclude that the combination of the anti-HIV-1 monoclonal antibodies 3BNC117 and 10-1074 can maintain long-term suppression in the absence of antiretroviral therapy in individuals with antibody-sensitive viral reservoirs.
Additional documents
No documents were uploaded
Useful links
There are no additional links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Not provided