
|
Developed by 

|
Supported by 

|
Ultra Long-Acting Tenofovir ProTide
Developer(s)

|
Exavir Therapeutics Originator
https://exavirtherapeutics.com/
United States Exavir Therapeutics is a biopharmaceutical company focused on developing ultra-long-acting therapeutics for chronic viral infections and CNS disorders. Headquartered in San Francisco, CA, they utilise prodrug nano-formulation technology to extend the half-life of drugs. Their current research focus primarily targets HIV, with the goal of improving treatment adherence and patient outcomes. |
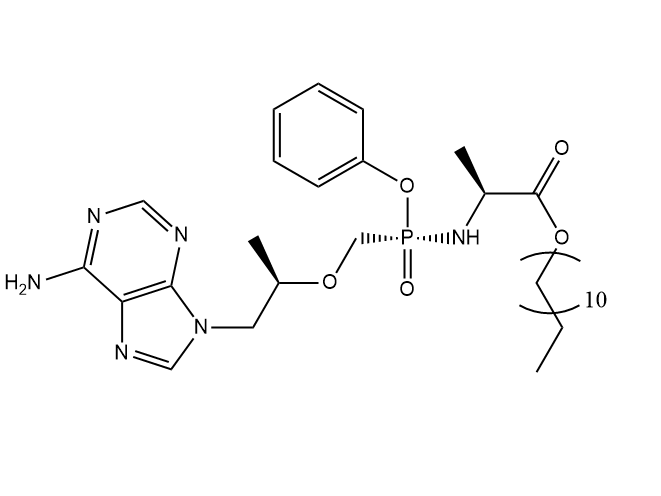
Drug structure

TFV ProTide Chemical Structure
Adapted from https://doi.org/10.1038/s41467-021-25690-5
Drug information
Associated long-acting platforms
Aqueous drug particle suspension, Solid Drug Nanoparticles
Administration route
Intramuscular
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
User acceptance
Not provided
Dosage
Available dose and strength
Not provided
Frequency of administration
Not provided
Maximum dose
Not provided
Recommended dosing regimen
The preclinical NM1TFV formulation suppressed HBV replication for 3 months after a single parenteral injection.
Additional comments
Not provided
Dosage link(s)
Not provided
Drug information
Drug's link(s)
Generic name
Brand name
Compound type
Summary
Approval status
Regulatory authorities
Delivery device(s)
No delivery device
Scale-up and manufacturing prospects
Scale-up prospects
Production scale-up viability for NM1TFV solid drug nanoformulations by high-pressure homogenization and/or wet bead milling processes have been established.
Tentative equipment list for manufacturing
Not provided
Manufacturing
Lipophilic ProTides of TFV bearing phenylalanine and alanine amino acid esters were created by replacing optimal short chain alkyl ester groups utilised by conventional ProTide strategies. The docosanol masking ester motif was selected based on inherent lipophilicity and synergy with nucleoside analogs. NM1TFV was synthesised by coupling a phenylalanyl or alanyl docosyl ester to monophenyl TFV in the presence of Et3N. As the conjugation step is moisture sensitive, further improvements to the chemical yields could be achieved by either optimizing the coupling reagents or reaction vessels.
Specific analytical instrument required for characterization of formulation
Dynamic light scattering (DLS) and HPLC to measure nanoparticle size, Autoflex maX MALDI-TOF/TOF mass spectrometer to confirm molecular mass.
Clinical trials
Not providedExcipients
Proprietary excipients used
No proprietary excipient used
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
No novel excipient or existing excipient used
Residual solvents used
Not provided
Patent info
There are either no relevant patents or these were not yet submitted to LAPaL
Supporting material
Publications
Srijanee Das et al., An ultralong-acting tenofovir ProTide nanoformulation achieves monthslong HBV suppression. Sci. Adv. 8, eade9582(2022). DOI:10.1126/sciadv.ade9582
Treatment of chronic hepatitis B virus (HBV) requires lifelong daily therapy. However, suboptimal adherence to the existing daily therapy has led to the need for ultralong-acting antivirals. A lipophilic and hydrophobic ProTide was made by replacing the alanyl isopropyl ester present in tenofovir alafenamide (TAF) with a docosyl phenyl alanyl ester, now referred to as M1TFV. NM1TFV and nanoformulated TAF (NTAF) nanocrystals were formulated by high-pressure homogenization. A single intramuscular injection of NM1TFV, but not NTAF, delivered at a dose of TFV equivalents (168 milligrams per kilogram) demonstrated monthslong antiviral activities in both HBV-transgenic and human hepatocyte transplanted TK-NOG mice. The suppression of HBV DNA in blood was maintained for 3 months. Laboratory experiments on HBV-transfected HepG2.2.15 cells affirmed the animal results and the critical role of docosanol in the sustained NM1TFV antiviral responses. These results provide clear “proof of concept” toward an emerging therapeutic paradigm for the treatment and prevention of HBV infection.
Cobb, D.A., Smith, N., Deodhar, S. et al. Transformation of tenofovir into stable ProTide nanocrystals with long-acting pharmacokinetic profiles. Nat Commun 12, 5458 (2021). DOI: https://doi.org/10.1038/s41467-021-25690-5
Treatment and prevention of human immunodeficiency virus type one (HIV-1) infection was transformed through widespread use of antiretroviral therapy (ART). However, ART has limitations in requiring life-long daily adherence. Such limitations have led to the creation of long-acting (LA) ART. While nucleoside reverse transcriptase inhibitors (NRTI) remain the ART backbone, to the best of our knowledge, none have been converted into LA agents. To these ends, we transformed tenofovir (TFV) into LA surfactant stabilized aqueous prodrug nanocrystals (referred to as NM1TFV and NM2TFV), enhancing intracellular drug uptake and retention. A single intramuscular injection of NM1TFV, NM2TFV, or a nanoformulated tenofovir alafenamide (NTAF) at 75 mg/kg TFV equivalents to Sprague Dawley rats sustains active TFV-diphosphate (TFV-DP) levels ≥ four times the 90% effective dose for two months. NM1TFV, NM2TFV and NTAF elicit TFV-DP levels of 11,276, 1,651, and 397 fmol/g in rectal tissue, respectively. These results are a significant step towards a LA TFV ProTide.
Additional documents
No documents were uploaded
Useful links
There are no additional links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Not provided