
|
Developed by 

|
Supported by 

|
Budigalimab
Developer(s)

|
AbbVie Inc. Originator
https://www.abbvie.co.uk/
United States of America AbbVie Inc. is an American pharmaceutical company headquartered in North Chicago, Illinois. It is a global biopharmaceutical company known for its focus on developing treatments in several therapeutic areas, including immunology, oncology, neuroscience, and virology. It was founded in 2013 as a spin-off from Abbott Laboratories to focus specifically on pharmaceuticals. |
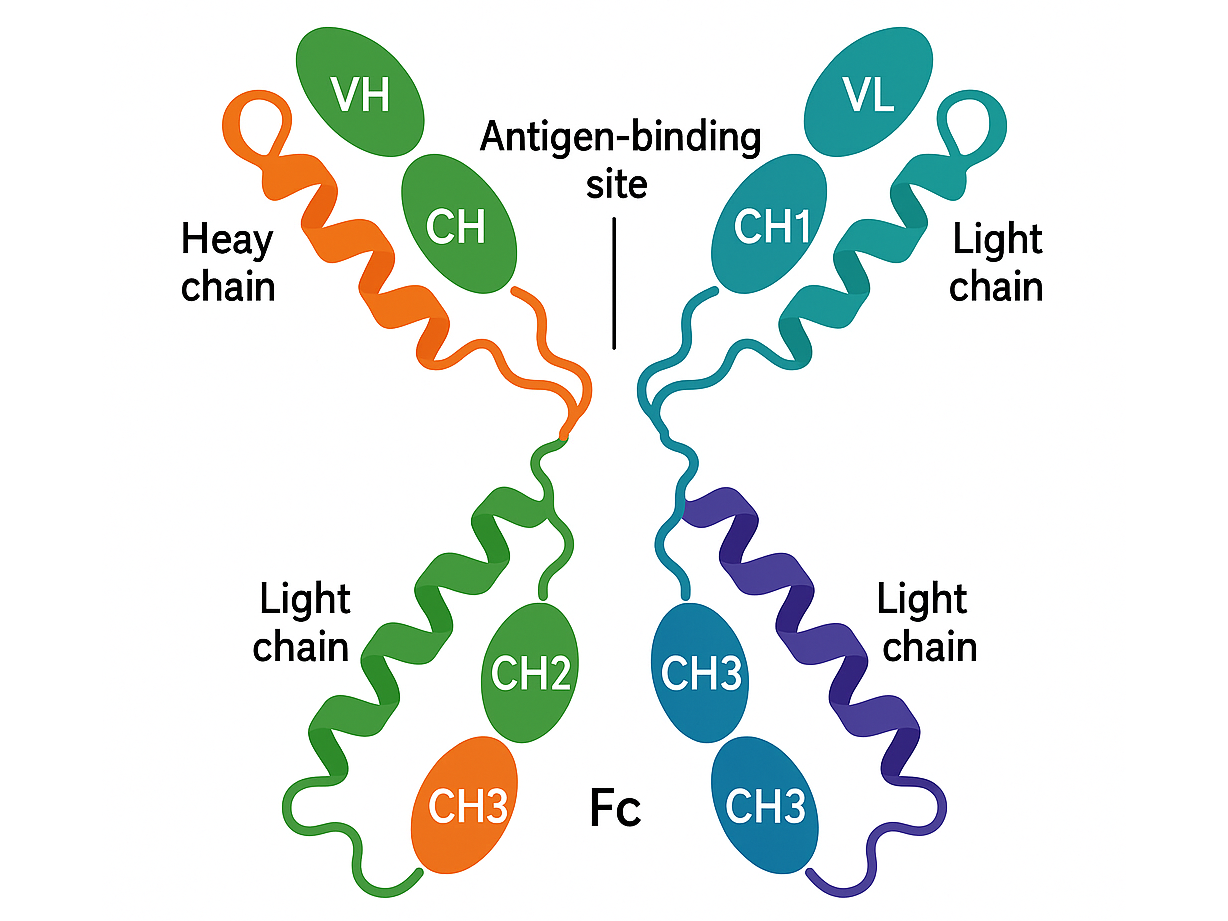
Drug structure

Budigalimab - a 3D drug structure with antigen binding site and Fc fragments.
MedChemExpress. (n.d.). Budigalimab (ABBV 181) | PD-1 receptor inhibitor. Retrieved April 17, 2025, from https://www.medchemexpress.com/budigalimab.html
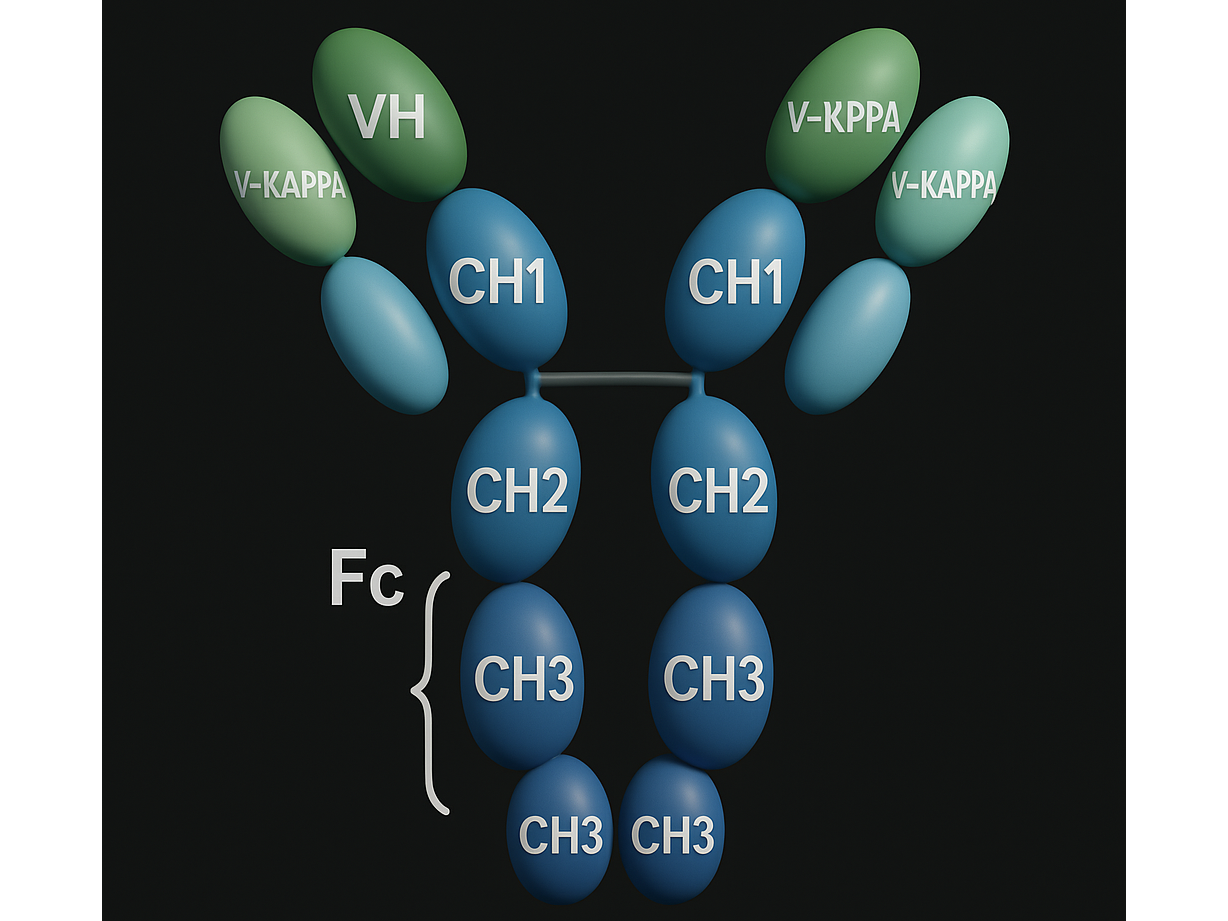
Humanized, recombinant IgG1 monoclonal antibody targeting programmed cell death 1 (PD-1) receptor
MedChemExpress. (n.d.). Budigalimab (ABBV 181) | PD-1 receptor inhibitor. Retrieved April 17, 2025, from https://www.medchemexpress.com/budigalimab.html
Drug information
Associated long-acting platforms
Solution
Administration route
Intravenous
Therapeutic area(s)
Use case(s)
Use of drug
Ease of administration
User acceptance
Not provided
Dosage
Available dose and strength
250mg and 500mg
Frequency of administration
Every 2 weeks; Every 4 weeks
Maximum dose
Not available
Recommended dosing regimen
Budigalimab 250 mg intravenously Q2W Budigalimab 500 mg intravenously Q4W
Additional comments
Not provided
Dosage link(s)
Not provided
Drug information
Drug's link(s)
Not provided
Generic name
Brand name
Compound type
Summary
Approval status
Regulatory authorities
Delivery device(s)
No delivery device
Scale-up and manufacturing prospects
Scale-up prospects
Not provided
Tentative equipment list for manufacturing
Not provided
Manufacturing
• Cell Line Development using CHO (Chinese Hamster Ovary) cell line. • Upstream Processing (USP): Cell Culture & Fermentation in bioreactors • Downstream Processing (DSP): Purification using Ion-Exchange Chromatography • Size exclusion chromatography (SEC) to remove aggregates and fragments
Specific analytical instrument required for characterization of formulation
• SEC-HPLC, SDS-PAGE, Mass spectrometry, peptide mapping
Clinical trials
M19-972
Identifier
NCT04799353
Link
https://clinicaltrials.gov/study/NCT04799353
Phase
Phase I
Status
Completed
Sponsor
AbbVie
More details
This study will evaluate how safe Budigalimab is and how it moves within the body in adult participants with HIV-1 infection. Budigalimab is an investigational drug being evaluated for the treatment of Human Immunodeficiency Virus. Study participants will be assigned to one of the 4 treatment groups and will receive a single dose of Budigalimab or placebo subcutaneous (SC) and intravenous (IV). Around 32 participants 18-65 years of age living with Human Immunodeficiency Virus will be enrolled in the study in approximately 9 sites worldwide. Each participant will receive single dose of SC and IV Budigalimab and/or Placebo on day 1 and will be followed for 24 weeks. Participants will attend weekly to every two and every four weeks visits during the study at a hospital. The effect of the t
Purpose
Study to Evaluate the Safety and How the Body Handles a Single Dose of Subcutaneous (SC) and Intravenous (IV) Budigalimab in Adult Participants Living With Human Immunodeficiency Virus (HIV)
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-03-15
Anticipated Date of Last Follow-up
2022-10-14
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-10-11
Actual Completion Date
2022-10-11
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Condition of generally good health, body mass index ≥ 18.0 to \< 35.0 kg/m2. * Laboratory values must meet acceptable criteria. * Human Immunodeficiency Virus (HIV-1) infected on antiretroviral therapy (ART) for at least 12 months prior to screening and on current ART regimen for at least 8 weeks prior to screening. * CD4 cell count ≥ 450 cells/μL at Screening and during the 12 months prior to Screening. * Plasma HIV-1 RNA below the lower limit of quantification at Screening and at least 6 months prior to Screening. * Participants agreeing to use an effective barrier method of protection (male and/or female condom) during sexual activity from Study Day 1 through last study visit for the purposes of prevention of HIV transmission. Exclusion Criteria: * Participants
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
33
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
M19-965
Identifier
NCT06032546
Link
https://clinicaltrials.gov/study/NCT06032546
Phase
Phase II
Status
Not provided
Sponsor
AbbVie
More details
Human immuno-deficiency virus (HIV) is the virus that causes Acquired Immuno-Deficiency Syndrome (AIDS). HIV disease is considered to be a chronic disease requiring lifelong therapy. The purpose of this study is to assess change in disease activity, adverse events, tolerability, and how the drug moves through the body. Budigalimab and ABBV-382 are investigational drugs being developed for the treatment of HIV disease. In Part 1, participants are placed in 1 of 5 groups, called treatment arms. Each group receives a different treatment. There is a 1 in 7 chance that participants will be assigned to placebo (A placebo is not a drug and it is not expected to have any chemical effects on your body and it is not designed to treat any disease or illness). In Part 2, eligible participants will be
Purpose
A Study to Assess Change in Disease Activity, Adverse Events, and How the Drug Moves Through the Body in Adult Participants Living With Human Immunodeficiency Virus (HIV) Receiving Intravenous (IV) In
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-10-12
Anticipated Date of Last Follow-up
2025-01-15
Estimated Primary Completion Date
2027-03-01
Estimated Completion Date
2027-03-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * A condition of general good health in the opinion of the investigator, based upon the results of a medical history, physical examination, vital signs, laboratory profile, and a 12-lead electrocardiogram (ECG). * Must be on antiretroviral therapy (ART) for at least 12 months prior to screening and on a stable ART regimen for at least 8 weeks prior to screening (current ART regimen cannot include an Non-nucleoside reverse transcriptase inhibitor \[NNRTI\] or long-acting ART). * Negative human immuno-deficiency virus (HIV)-2 antibody (Ab) * Cluster of differentiation 4 (CD4+) T cell count \>= 500 cells/μL at screening and no known evidence of CD4+ T cell count \< 500 cells/μL in the last 12 months prior to screening * Participant must have plasma HIV-1 ribonucleic acid
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
163
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Not provided
Studied LA-formulation(s)
Not provided
Studied route(s) of administration
Not provided
Use case
Not provided
Key resources
M24-695
Identifier
NCT06487559
Link
https://clinicaltrials.gov/study/NCT06487559
Phase
Phase I
Status
Recruiting
Sponsor
AbbVie
More details
Hepatocellular carcinoma (HCC) is a common cancer worldwide and a leading cause of cancer-related death. The majority of participants first presenting with HCC have advanced unresectable or metastatic disease. The purpose of this study is to assess adverse events and how livmoniplimab in combination with budigalimab moves through the body in adult Chinese participants with Locally Advanced or metastatic Child-Pugh A Hepatocellular Carcinoma (HCC). Livmoniplimab is an investigational drug being developed for the treatment of HCC. There are 2 stages to this study. Stage 1 is a safety run-in. There are 2 treatment arms in stage 1 and participants will receive escalating doses of Livmoniplimab in combination with budigalimab (fixed dose). Stage 2 is dose expansion. There are 2 treatment arms
Purpose
A Study to Assess the Adverse Events and How Intravenously Infused Livmoniplimab in Combination With Budigalimab Moves Through the Bodies of Adult Chinese Participants With Locally Advanced or Metasta
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2024-09-11
Anticipated Date of Last Follow-up
2025-02-28
Estimated Primary Completion Date
2027-10-01
Estimated Completion Date
2027-10-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Locally advanced or metastatic and/or unresectable HCC * Child-Pugh A * Barcelona Clinic Liver Cancer stage B or C * Eastern Cooperative Oncology Group (ECOG) Perfromance Status of 0-1 * Received an immune checkpoint inhibitor in 1L HCC treatment regimen * Adequate hematologic and end-organ function Exclusion Criteria: * Symptomatic, untreated, or actively progressing central nervous system (CNS) metastases as outlined in the protocol. * History of malignancy other than HCC within 5 years prior to screening, except for malignancies with a negligible risk of metastasis or death (e.g., 5-year OS rate \> 90%). * History of autoimmune, immune deficiency, or inflammatory disorders including, but not limited to, inflammatory bowel disease, systemic lupus erythematosus, s
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
20
Allocation
Not provided
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
M21-410
Identifier
NCT05005403
Link
https://clinicaltrials.gov/study/NCT05005403
Phase
Phase I
Status
Recruiting
Sponsor
AbbVie
More details
Cancer is a condition where cells in a specific part of body grow and reproduce uncontrollably. Non-Small Cell Lung Cancer (NSCLC) is a solid tumor, a disease in which cancer cells form in the tissues of the lung. Head and Neck Squamous Cell Carcinoma (HNSCC) is a solid tumor, a disease in which cancer cells form in the tissues of the head and neck. The purpose of this study is to assess adverse events and pharmacokinetics of ABBV-514 as a monotherapy and in combination with Budigalimab. Budigalimab and ABBV-514 are investigational drugs being developed for the treatment of NSCLC, HNSCC, and other solid tumors. Study doctors put the participants in groups called treatment arms. The maximum-tolerated dose (MTD)/maximum administered dose (MAD) of ABBV-514 will be explored. Each treatment ar
Purpose
Study to Assess Adverse Events and Pharmacokinetics in Adult Participants With Non-Small Cell Lung Cancer (NSCLC), Head and Neck Squamous Cell Carcinoma (HNSCC) and Other Solid Tumors, Receiving Intra
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-11-01
Anticipated Date of Last Follow-up
2025-01-27
Estimated Primary Completion Date
2026-05-01
Estimated Completion Date
2026-05-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Dose-escalation cohorts only: -- Must have an advanced solid tumor who are considered refractory to or intolerant of all existing therapies known to provide a clinical benefit for their condition. * Relapsed Non-Small Cell Lung Cancer (NSCLC) Head and Neck Squamous Cell Carcinoma (HNSCC) dose-expansion cohorts only: * Must have histologically or cytologically confirmed advanced or metastatic NSCLC or HNSCC that is not suitable for surgical resection and / or radiation therapy and has been treated with platinum-based chemotherapy and a programmed cell death (PD)-1 or PD ligand 1 (PD-L1) targeting agent (separately or in combination therapy). * Must have failed (or refused) treatment with available therapies known to be active for treatment of their disease. *
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
268
Allocation
Not provided
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
M19-345
Identifier
NCT03821935
Link
https://clinicaltrials.gov/study/NCT03821935
Phase
Phase I
Status
Recruiting
Sponsor
AbbVie
More details
The study will determine the recommended Phase 2 dose (RP2D) of livmoniplimab (ABBV-151) administered as monotherapy and in combination with budigalimab (ABBV-181) as well as to assess the safety, tolerability, pharmacokinetics (PK), and preliminary efficacy of livmoniplimab alone and in combination with budigalimab. The study will consist of 2 parts: dose escalation and dose expansion.
Purpose
Study to Determine the Safety, Tolerability, Pharmacokinetics and Recommended Phase 2 Dose (RP2D) of Livmoniplimab (ABBV-151) as a Single Agent and in Combination With Budigalimab (ABBV-181) in Partic
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-02-21
Anticipated Date of Last Follow-up
2025-04-17
Estimated Primary Completion Date
2027-06-01
Estimated Completion Date
2027-06-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * For Dose Escalation only: Participants with an advanced solid tumor who are considered refractory to or intolerant of all existing therapy(ies) known to provide a clinical benefit for their condition. Additionally, participants who have been offered standard therapies and refused, or who are considered ineligible for standard therapies, may be eligible for this study on a case-by-case basis, after discussion with and agreement from the sponsor. Participants with pancreatic adenocarcinoma, urothelial cancer (UC), hepatocellular carcinoma (HCC), or head and neck squamous cell carcinoma (HNSCC) who are being considered for the dose escalation cohorts must also meet the histology specific eligibility criteria described below for dose expansion. * For Dose Expansion only
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
362
Allocation
Randomized
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Not provided
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
M19-345
Identifier
NCT03821935
Link
https://clinicaltrials.gov/study/NCT03821935
Phase
Phase I
Status
Recruiting
Sponsor
AbbVie
More details
The study will determine the recommended Phase 2 dose (RP2D) of livmoniplimab (ABBV-151) administered as monotherapy and in combination with budigalimab (ABBV-181) as well as to assess the safety, tolerability, pharmacokinetics (PK), and preliminary efficacy of livmoniplimab alone and in combination with budigalimab. The study will consist of 2 parts: dose escalation and dose expansion.
Purpose
Study to Determine the Safety, Tolerability, Pharmacokinetics and Recommended Phase 2 Dose (RP2D) of Livmoniplimab (ABBV-151) as a Single Agent and in Combination With Budigalimab (ABBV-181) in Partic
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-02-21
Anticipated Date of Last Follow-up
2025-04-17
Estimated Primary Completion Date
2027-06-01
Estimated Completion Date
2027-06-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * For Dose Escalation only: Participants with an advanced solid tumor who are considered refractory to or intolerant of all existing therapy(ies) known to provide a clinical benefit for their condition. Additionally, participants who have been offered standard therapies and refused, or who are considered ineligible for standard therapies, may be eligible for this study on a case-by-case basis, after discussion with and agreement from the sponsor. Participants with pancreatic adenocarcinoma, urothelial cancer (UC), hepatocellular carcinoma (HCC), or head and neck squamous cell carcinoma (HNSCC) who are being considered for the dose escalation cohorts must also meet the histology specific eligibility criteria described below for dose expansion. * For Dose Expansion only
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
362
Allocation
Randomized
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Not provided
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
M24-122
Identifier
NCT06158958
Link
https://clinicaltrials.gov/study/NCT06158958
Phase
Phase I
Status
Recruiting
Sponsor
AbbVie
More details
Cancer is a condition where cells in a specific part of body grow and reproduce uncontrollably. The purpose of this study is to assess safety, tolerability, pharmacokinetics and preliminary efficacy of ABBV-303 as a monotherapy and in combination with budigalimab, (ABBV-181). ABBV-303 is an investigational drug being developed for the treatment of solid tumors. There are multiple treatment arms in this study. Participants will either receive ABBV-303 as a single agent or in combination with budigalimab (another investigational drug) at different doses. Approximately 181 adult participants will be enrolled in the study across sites worldwide. In Part A, ABBV-303 will be intravenously (IV) infused in escalating doses as a monotherapy in participants with relapsed (R)/refractory (R) solid t
Purpose
A Study to Assess the Safety, Pharmacokinetics, and Efficacy of Intravenous (IV) ABBV-303, as Monotherapy and in Combination With IV Infused Budigalimab (ABBV-181), in Adults With Advanced Solid Tumor
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2024-02-06
Anticipated Date of Last Follow-up
2024-10-07
Estimated Primary Completion Date
2028-01-20
Estimated Completion Date
2028-01-20
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Participants with Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. * Laboratory values meeting the protocol's criteria within the screening period (-28 days) prior to the first dose of study drug. * Participants with a diagnosis of a malignant solid tumor by histology (World Health Organization \[WHO\] criteria). * Participants with measurable disease per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1). Exclusion Criteria: * Unresolved Grade \> 1 adverse events (AEs) from prior anti-cancer therapy except for alopecia. * Active systemic or uncontrolled local bacterial, fungal, or viral infection requiring antimicrobial therapy. * History of hypersensitivity to the active ingredients or any excipients of ABBV-303 and budi
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
192
Allocation
Not provided
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Not provided
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
M17-327
Identifier
NCT03639194
Link
https://clinicaltrials.gov/study/NCT03639194
Phase
Phase I
Status
Completed
Sponsor
AbbVie
More details
This is a multicenter, open-label, Phase 1 study of ABBV-011 given as a single agent and in combination with budigalimab (ABBV-181) in participants with relapsed or refractory small cell lung cancer (SCLC). The study consists of 4 parts: Part A is a single-agent ABBV-011 dose regimen finding cohort; followed by Part B, a single-agent ABBV-011 dose expansion cohort; and then Part C, an ABBV-011 and budigalimab (ABBV-181) combination escalation and expansion cohort; Part D, single-agent ABBV-011 dose-evaluating cohort for Japan.
Purpose
A Study of ABBV-011 Alone and in Combination With Budigalimab (ABBV-181) in Participants With Relapsed or Refractory Small Cell Lung Cancer
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2018-10-24
Anticipated Date of Last Follow-up
2024-02-08
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2024-01-25
Actual Completion Date
2024-01-25
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Histologically or cytologically confirmed small cell lung cancer (SCLC) that is relapsed or refractory following at least 1 prior platinum-containing chemotherapy, but no more than 3 total prior lines of therapy, and with no curative therapy available. * Measurable disease, defined as at least 1 tumor lesion greater than or equal to 10 mm in the longest diameter or a lymph node greater than or equal to 15 mm in short axis measurement assessed by computed tomography (CT) scan, according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. * Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. * Minimum life expectancy of at least 12 weeks. * Recovery to at least Grade 1 of any clinically significant toxicity (excluding alopecia) pri
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
132
Allocation
Not provided
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
LIVIGNO-1
Identifier
NCT05822752
Link
https://clinicaltrials.gov/study/NCT05822752
Phase
Phase II
Status
Not provided
Sponsor
AbbVie
More details
Hepatocellular carcinoma (HCC) is a common cancer worldwide and a leading cause of cancer-related death. The majority of participants first presenting with HCC have advanced unresectable or metastatic disease. The purpose of this study is to evaluate the optimized dose, adverse events, and efficacy of livmoniplimab in combination with budigalimab. Livmoniplimab is an investigational drug being developed for the treatment of HCC. There are 3 treatment arms in this study and participants will be randomized in a 1:1:1 ratio. Participants will either receive livmoniplimab (at different doses) in combination with budigalimab (another investigational drug), lenvatinib, or sorafenib. Approximately 120 adult participants will be enrolled in the study across 60 sites worldwide. In arm 1 (control)
Purpose
Study to Evaluate Adverse Events, and Change in Disease Activity, When Intravenously (IV) Infused With Livmoniplimab in Combination With IV Infused Budigalimab in Adult Participants With Hepatocellula
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-09-21
Anticipated Date of Last Follow-up
2025-01-02
Estimated Primary Completion Date
2026-11-01
Estimated Completion Date
2026-11-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Child-Pugh A classification. * Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 to 1. * Received an immune checkpoint inhibitor in first-line (1L) hepatocellular carcinoma (HCC) treatment regimen. * Adequate hematologic and end-organ function. * Tissue biopsy at screening. * Disease that is not amenable to surgical and/or locoregional therapies, or progressive disease after surgical and /or locoregional therapies. Exclusion Criteria: * Symptomatic, untreated, or actively progressing central nervous system (CNS) metastases. * Prior treatment with an approved tyrosine kinase inhibitor (for example sorafenib or Lenvatinib) in 1L HCC treatment regimen. * History of malignancy other than hepatocellular carcinoma (HCC) within 5 years prior to screening.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
130
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
M19-037
Identifier
NCT03893955
Link
https://clinicaltrials.gov/study/NCT03893955
Phase
Phase I
Status
Not provided
Sponsor
AbbVie
More details
A study evaluating the safety, pharmacokinetics (PK), pharmacodynamics, and preliminary efficacy of ABBV-927 with ABBV-368, Budigalimab (ABBV-181) and/or chemotherapy in participants with selected solid tumors. This study consists of 2 main parts, a dose-escalation phase and a dose-expansion phase. The dose-expansion phase can begin once the recommended phase 2 dose/maximum tolerated dose (RP2D/MTD) is determined in the dose-escalation phase.
Purpose
A Study to Determine the Safety, Tolerability, Pharmacokinetics, and Preliminary Efficacy of ABBV-927 With ABBV-368, Budigalimab (ABBV-181) and/or Chemotherapy in Participants With Locally Advanced or
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-05-21
Anticipated Date of Last Follow-up
2024-08-12
Estimated Primary Completion Date
2025-03-30
Estimated Completion Date
2025-03-30
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Adequate liver, kidney and hematology function as demonstrated by laboratory values detailed in the study protocol. * An Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Dose-Escalation: * Arm A: Participants with an advanced solid tumor who have progressed on standard therapies known to provide clinical benefit and/or participants who have refused or are intolerant of such therapy. * Arm B (non-small-cell-lung-cancer \[NSCLC\]): Participants with histologically or cytologically confirmed NSCLC who previously progressed during or after an anti-programmed cell death (PD)-1 or PD ligand 1 (PD-L1) therapy and a platinum-based regimen in the recurrent or metastatic setting. Dose-Expansion: * Arm 1, 2, and 3 (triple-negative breast cancer \[TNBC
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
150
Allocation
Not provided
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Not provided
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
M19-939
Identifier
NCT04223804
Link
https://clinicaltrials.gov/study/NCT04223804
Phase
Phase I
Status
Completed
Sponsor
AbbVie
More details
This study will be conducted in two stages and will test the safety/tolerability, pharmacokinetics (how the body handles study drug) and pharmacodynamics (effects on the immune system and the virus) of the study drug ABBV-181 in Human immunodeficiency virus (HIV)-1 infected participants undergoing Antiretroviral therapy (ART) interruption.
Purpose
A Study to Evaluate the Safety, Pharmacokinetics and Pharmacodynamics of ABBV-181 (Budigalimab) in Adult Participants With Human Immunodeficiency Virus (HIV)-1
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2020-01-30
Anticipated Date of Last Follow-up
2023-03-07
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2023-02-27
Actual Completion Date
2023-02-27
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Body Mass Index (BMI) between 18.0 and 35 kg/m2. * HIV-1 infected on antiretroviral therapy (ART) for at least 12 months prior to screening and on current ART regimen for at least 8 weeks prior to screening. * Meets HIV-specific laboratory parameters as below: * Plasma HIV-1 RNA below lower limit of quantification (LLOQ) at screening and at least 6 months prior to screening. * CD4+ T cell count \>= 500 cells/uL at screening and at least once during the 12 months prior to screening. * CD4+ T cell nadir of \>= 200 cells/uL during chronic infection. * Willing to undergo ART interruption. * Agrees to use an effective barrier method of protection (male and/or female condoms) during sexual activity for protection against HIV-1 transmission throughout the entire stud
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
41
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Not provided
Frequency of administration
Not provided
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
M15-891
Identifier
NCT03000257
Link
https://clinicaltrials.gov/study/NCT03000257
Phase
Phase I
Status
Completed
Sponsor
AbbVie
More details
This is an open-label, Phase I, dose-escalation study to determine the recommended Phase 2 dose (RPTD), maximum tolerated dose (MTD), and evaluate the safety and pharmacokinetic (PK) profile of budigalimab. This study will also evaluate the safety and tolerability of budigalimab in combination with Rovalpituzumab Tesirine and budigalimab in combination with venetoclax. The study will consist of 3 parts: budigalimab monotherapy dose escalation and expansion, budigalimab in combination with Rovalpituzumab Tesirine and budigalimab in combination with venetoclax.
Purpose
A Study of Budigalimab (ABBV-181) in Participants With Advanced Solid Tumors
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2016-12-14
Anticipated Date of Last Follow-up
2022-04-13
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-03-29
Actual Completion Date
2022-03-29
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Participant must have an advanced solid tumor and must not be a candidate for surgical resection or other approved therapeutic regimen known to provide clinical benefit. For dose escalation, the participant may have been previously treated with a programmed cell death 1 (PD-I) targeting agent. For dose expansion, the participant must be PD-I/PD-L1 targeting agent naïve. For Part 2 budigalimab in combination with rovalpituzumab tesirine, the participant must have SCLC with progressive disease and have failed platinum containing therapy and be PD-1/PD-L1 targeting agent naïve. For Part 3 budigalimab in combination with venetoclax, the participant must have locally advanced or metastatic NSCLC and received 1 to 4 prior lines of therapy in the advanced or metastatic sett
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
182
Allocation
Not provided
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
M15-891
Identifier
NCT03000257
Link
https://clinicaltrials.gov/study/NCT03000257
Phase
Phase I
Status
Completed
Sponsor
AbbVie
More details
This is an open-label, Phase I, dose-escalation study to determine the recommended Phase 2 dose (RPTD), maximum tolerated dose (MTD), and evaluate the safety and pharmacokinetic (PK) profile of budigalimab. This study will also evaluate the safety and tolerability of budigalimab in combination with Rovalpituzumab Tesirine and budigalimab in combination with venetoclax. The study will consist of 3 parts: budigalimab monotherapy dose escalation and expansion, budigalimab in combination with Rovalpituzumab Tesirine and budigalimab in combination with venetoclax.
Purpose
A Study of Budigalimab (ABBV-181) in Participants With Advanced Solid Tumors
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2016-12-14
Anticipated Date of Last Follow-up
2022-04-13
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-03-29
Actual Completion Date
2022-03-29
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Participant must have an advanced solid tumor and must not be a candidate for surgical resection or other approved therapeutic regimen known to provide clinical benefit. For dose escalation, the participant may have been previously treated with a programmed cell death 1 (PD-I) targeting agent. For dose expansion, the participant must be PD-I/PD-L1 targeting agent naïve. For Part 2 budigalimab in combination with rovalpituzumab tesirine, the participant must have SCLC with progressive disease and have failed platinum containing therapy and be PD-1/PD-L1 targeting agent naïve. For Part 3 budigalimab in combination with venetoclax, the participant must have locally advanced or metastatic NSCLC and received 1 to 4 prior lines of therapy in the advanced or metastatic sett
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
182
Allocation
Not provided
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
M23-385
Identifier
NCT05599984
Link
https://clinicaltrials.gov/study/NCT05599984
Phase
Phase I
Status
Recruiting
Sponsor
AbbVie
More details
Cancer is a condition where cells in a specific part of body grow and reproduce uncontrollably. The purpose of this study is to assess safety, tolerability, pharmacokinetics and preliminary efficacy of ABBV-706 as a monotherapy and in combination with budigalimab, carboplatin, or cisplatin. ABBV-706 is an investigational drug being developed for the treatment of small cell lung cancer (SCLC), high-grade central nervous system (CNS) tumors and high-grade neuroendocrine carcinomas (NECs). There are multiple treatment arms in this study. Participants will either receive ABBV-706 as a single agent or in combination with budigalimab (another investigational drug), carboplatin or cisplatin at different doses. Approximately 350 adult participants will be enrolled in the study across sites worldw
Purpose
Study to Evaluate Adverse Events, Change in Disease Activity, and How ABBV-706 Moves Through the Body When Intravenously (IV) Infused Alone or in Combination With IV Infused Budigalimab, Cisplatin, or
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-12-05
Anticipated Date of Last Follow-up
2025-03-24
Estimated Primary Completion Date
2027-11-01
Estimated Completion Date
2027-11-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. * The laboratory values criteria must be met within 7 days prior to the first dose of study drug as per the protocol. * QT interval corrected for heart rate (QTc) \<= 450 msec (males) or \<= 470 msec (females) using Fridericia's correction, and an ejection fraction of \>= 50% as measured by echocardiogram or multigated acquisition (MUGA) scan at Screening. * Part 1 only: Advanced recurrent or refractory solid tumors with potential SEZ6 expression including small cell lung cancer (SCLC), high-grade central nervous system (CNS) tumors (glioblastoma \[GBM\], IDH-wildtype Grade 4; oligodendroglioma, IDH-mutant, and 1p/19q-codeleted Grade 3; astrocytoma, IDH-mutant Grade 3 or Grade 4), neuroendocrine
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
350
Allocation
Not provided
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
M15-862
Identifier
NCT02988960
Link
https://clinicaltrials.gov/study/NCT02988960
Phase
Phase I
Status
Not provided
Sponsor
AbbVie
More details
This is a dose-escalation study designed to evaluate the safety, pharmacokinetics, and pharmacodynamics of ABBV-927, and to determine the maximum tolerated dose (MTD) or recommended Phase 2 dose (RPTD) for ABBV-927 when administered as monotherapy or as combination therapy with ABBV-181 in participants with advanced solid tumors.
Purpose
A Study of ABBV-927 and ABBV-181, an Immunotherapy, in Participants With Advanced Solid Tumors
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2017-02-22
Anticipated Date of Last Follow-up
2024-12-16
Estimated Primary Completion Date
2025-09-01
Estimated Completion Date
2025-09-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Participant has an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 to 1. * Participants have adequate bone marrow, kidney and liver function. * Participants with a history of chronic heart failure or significant cardiovascular disease must have an echocardiogram or multigated acquisition scan indicating left ventricular ejection fraction greater than or equal to 45% within 28 days prior to the first dose of study drug. * Participants must have creatinine clearance greater than or equal to 50 mL/min as measured by 24-hour urine or estimated by the Cockcroft-Gault formula. * Participants must have total bilirubin less than or equal to 1.5 times the upper limit of normal (ULN), and aspartate aminotransferase and alanine aminotransferase less than or eq
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
163
Allocation
Not provided
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
M19-894
Identifier
NCT04196283
Link
https://clinicaltrials.gov/study/NCT04196283
Phase
Phase I
Status
Completed
Sponsor
AbbVie
More details
The main objective of this study is to assess safety, tolerability, and pharmacokinetics (PK) of ABBV-368 plus tilsotolimod; ABBV-368 plus tilsotolimod and nab-paclitaxel; and ABBV-368 plus tilsotolimod, nab-paclitaxel, and ABBV-181 in participants with recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC).
Purpose
A Study to Determine the Safety, Tolerability, Pharmacokinetics, and Preliminary Efficacy of ABBV-368 Plus Tilsotolimod and Other Therapy Combinations in Participants With Recurrent/Metastatic Head an
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2020-01-22
Anticipated Date of Last Follow-up
2023-02-24
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-10-27
Actual Completion Date
2022-10-27
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Participants should weigh at least 35 kg. * Eastern Cooperative Oncology Group performance status of 0 or 1 and a life expectancy of \>= 3 months. * Participant have \>= 1 lesion accessible for intratumoral injection. * Histologically or cytologically confirmed R/M HNSCC (of the following 4 subsites: oral cavity, oropharynx, larynx, and hypopharynx) who previously progressed either during or after \<= 3 prior treatment regimens administered in the recurrent or metastatic setting. * Must have received 1 immunotherapy regimen which included a PD-(L)1 inhibitor. * Must have received platinum-based therapy, or be considered ineligible for platinum-based therapy by the investigator. Exclusion Criteria: * Uncontrolled metastases to the central nervous system (CNS).
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
30
Allocation
Not provided
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
AndroMETa-GEA
Identifier
NCT06628310
Link
https://clinicaltrials.gov/study/NCT06628310
Phase
Phase II
Status
Recruiting
Sponsor
AbbVie
More details
Cancer is a condition where cells in a specific part of body grow and reproduce uncontrollably. The purpose of this study is to assess adverse events and change in disease activity when ABBV-400 is given in combination with Fluorouracil, Leucovorin, and a programmed cell death receptor 1 (PD1) inhibitor (Budigalimab) (AFLB) to adult participants to treat locally advanced unresectable or metastatic gastric, gastroesophageal junction, or esophageal adenocarcinoma (mGEA). ABBV-400 and Budigalimab are investigational drugs being developed for the treatment of mGEA. Fluorouracil and Leucovorin are drugs approved for the treatment of mGEA. This study will be divided into two stages, with the first stage treating participants with increasing doses of ABBV-400 within the AFLB regimen until the do
Purpose
A Study to Evaluate the Adverse Events, Efficacy, and Optimal Dose of Intravenous (IV) ABBV-400 in Combination With IV Fluorouracil, Leucovorin, and Budigalimab in Adult Participants With Locally Adva
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2024-12-13
Anticipated Date of Last Follow-up
2025-03-17
Estimated Primary Completion Date
2030-10-01
Estimated Completion Date
2030-10-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Have inoperable, advanced or metastatic histologically- or cytologically confirmed gastric, gastroesophageal junction, or esophageal adenocarcinoma. * Have measurable disease determined using Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. * Have an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1. * Human epidermal growth factor receptor 2 (HER2) negative disease, defined as immunohistochemistry (IHC) (0, or 1+) or fluorescence in situ hybridization (FISH) negative. * Known programmed death ligand 1 (PD-L1) status at screening, or availability of tumor tissue for local or central PD-L1 testing prior to enrollment. Exclusion Criteria: * Have prior systemic therapy in the locally advanced, unresectable, or metastatic setting. *
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
180
Allocation
Randomized
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
TTX-030-002
Identifier
NCT04306900
Link
https://clinicaltrials.gov/study/NCT04306900
Phase
Phase I
Status
Completed
Sponsor
Trishula Therapeutics, Inc.
More details
This is a phase 1/1b study of TTX-030 in combination therapy, an antibody that inhibits CD39 enzymatic activity, leading to accumulation of pro-inflammatory adenosine triphosphate (ATP) and reduction of immunosuppressive adenosine, which may change the tumor microenvironment and promote anti-tumor immune response. This trial will study the safety, tolerability, pharmacokinetics, pharmacodynamics and anti-tumor activity of TTX-030 in combination with immunotherapy and/or standard chemotherapies.
Purpose
TTX-030 in Combination With Immunotherapy and/or Chemotherapy in Subjects With Advanced Cancers
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2020-03-30
Anticipated Date of Last Follow-up
2024-04-04
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-11-30
Actual Completion Date
2024-03-27
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Abbreviated Inclusion Criteria: 1. Age 18 years or older, is willing and able to provide informed consent 2. Histologically confirmed diagnosis of unresectable or metastatic solid tumor malignancy in selected tumor types 3. Life expectancy \> 12 weeks 4. ECOG performance status of 0-1 Abbreviated Exclusion Criteria: 1. History of allergy or hypersensitivity to study treatment components. Patients with a history of severe hypersensitivity reaction to any monoclonal antibody. 2. Use of investigational agent within 28 days prior to the first dose of study treatment and throughout the study 3. Receiving high-dose systemic steroid therapy or any other form of immunosuppressive therapy 4. History of severe autoimmune disease 5. Uncontrolled intercurrent illness or other active malignancy requ
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
185
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Not provided
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
LIVIGNO-2
Identifier
NCT06109272
Link
https://clinicaltrials.gov/study/NCT06109272
Phase
Phase II/III
Status
Not provided
Sponsor
AbbVie
More details
Hepatocellular carcinoma (HCC) is a common cancer worldwide and a leading cause of cancer-related death. The majority of participants first presenting with HCC have advanced unresectable or metastatic disease. The purpose of this study is to evaluate the optimized dose, adverse events, and efficacy of livmoniplimab in combination with budigalimab. Livmoniplimab is an investigational drug being developed for the treatment of HCC. There are 2 stages to this study. In Stage 1, there are 3 treatment arms and participants will be randomized in a 1:1:1 ratio. Participants will either receive livmoniplimab (at different doses) in combination with budigalimab (another investigational drug), atezolizumab in combination with bevacizumab, or tremelimumab in combination with durvalumab. In Stage 2, t
Purpose
A Study to Assess the Dose, Adverse Events, and Change in Disease Activity of Livmoniplimab as an Intravenous (IV) Solution in Combination With Budigalimab as an IV Solution in Adult Participants With
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2024-01-11
Anticipated Date of Last Follow-up
2025-03-17
Estimated Primary Completion Date
2030-09-01
Estimated Completion Date
2030-09-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Locally advanced or metastatic and/or unresectable hepatocellular carcinoma (HCC) with diagnosis confirmed by histology or cytology or clinically by American Association for the Study of Liver Diseases criteria for participants with cirrhosis. * Barcelona Clinic Liver Cancer (BCLC) Stage B or C. * Child-Pugh A or B7 classification (i.e., total Child-Pugh score of 5, 6, or 7). * Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 to 1. Exclusion Criteria: * Prior systemic therapy for HCC. * Symptomatic, untreated, or actively progressing CNS metastases. * History of malignancy other than HCC.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
660
Allocation
Randomized
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
LIVIGNO-3
Identifier
NCT06632951
Link
https://clinicaltrials.gov/study/NCT06632951
Phase
Phase II
Status
Recruiting
Sponsor
AbbVie
More details
Urothelial carcinoma (UC) is the ninth most common cancer type worldwide. While the treatment of front-line metastatic urothelial carcinoma (mUC) has improved, there remains a high unmet need for effective therapies for participants who have recurrent disease and disease that has progressed after frontline treatment. The purpose of this study is to evaluate the optimized dose, adverse events, and efficacy of livmoniplimab in combination with budigalimab. Livmoniplimab is an investigational drug being developed for the treatment of mUC. There are 3 treatment arms in this study and participants will be randomized in a 1:1:1 ratio. Participants will either receive livmoniplimab (at one of 2 different doses) in combination with budigalimab (another investigational drug), or either docetaxel,
Purpose
Study to Evaluate Adverse Events and Change in Disease Activity When Intravenously (IV) Infused Livmoniplimab is Used in Combination With IV Infused Budigalimab in Adult Participants With Urothelial C
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2025-01-20
Anticipated Date of Last Follow-up
2025-04-17
Estimated Primary Completion Date
2028-08-01
Estimated Completion Date
2028-08-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Participant has histologically or cytologically confirmed urothelial carcinoma (i.e., cancer of the bladder, renal pelvis, ureter, or urethra). Mixed histologic types are allowed if urothelial (transitional cell) is the predominant histology. * Participant has radiologically documented metastatic disease. * Participant must have experienced radiographic progression or relapse on checkpoint inhibitor (anti-programmed cell death protein 1 \[PD-1\] or anti-programmed death-ligand 1 \[PD-L1\]) in the metastatic, adjuvant, or neo-adjuvant setting. Participant must have received at least 2 cycles of anti-PD-1 or anti-PD-L1. * Participants eligible for platinum must have received a platinum containing regimen (cisplatin or carboplatin) in the metastatic, locally advanced, n
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
150
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
LIVIGNO-4
Identifier
NCT06236438
Link
https://clinicaltrials.gov/study/NCT06236438
Phase
Phase II/III
Status
Recruiting
Sponsor
AbbVie
More details
Non-Squamous Non-Small Cell Lung Cancer (NSCLC) remains a leading cause of cancer mortality worldwide, with poor survival prospects for metastatic disease. The purpose of this study is to evaluate the optimized dose, adverse events, and efficacy of livmoniplimab in combination with budigalimab plus chemotherapy versus pembrolizumab plus chemotherapy in participants with untreated metastatic non-squamous non-small cell lung cancer. Livmoniplimab is an investigational drug being developed for the treatment of NSCLC. There are 2 stages to this study. In Stage 1, there are 4 treatment arms. Participants will either receive livmoniplimab (at different doses) in combination with budigalimab (another investigational drug) + chemotherapy, budigalimab +chemotherapy, or pembrolizumab +chemotherapy.
Purpose
Study to Evaluate Adverse Events, Optimal Dose, and Change in Disease Activity, With Livmoniplimab in Combination With Budigalimab Plus Chemotherapy Versus IV Infused Pembrolizumab Plus Chemotherapy i
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2024-04-10
Anticipated Date of Last Follow-up
2025-04-17
Estimated Primary Completion Date
2031-10-01
Estimated Completion Date
2031-10-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Diagnosis of histologically or cytologically confirmed metastatic nonsquamous non-small cell lung cancer (NSCLC) with no known epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK) mutation, or other genomic aberration for which a locally approved targeted therapy is available. * Must have at least 1 measurable lesion per response evaluation criteria in solid tumors (RECIST) v1.1 as determined by the local site Investigator/radiology assessment. * Life expectancy of at least 3 months and adequate organ function. Exclusion Criteria: - Received prior systemic therapy for the treatment of metastatic NSCLC.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
840
Allocation
Randomized
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
M24-536
Identifier
NCT06772623
Link
https://clinicaltrials.gov/study/NCT06772623
Phase
Phase I/II
Status
Recruiting
Sponsor
AbbVie
More details
Non small cell lung carcinoma (NSCLC) is the most frequently occurring histologic subtype of lung cancer and is the leading cause of cancer-related deaths worldwide. The purpose of this study is to assess adverse events and change in disease activity when Telisotuzumab Adizutecan (ABBV-400) is given in combination with a programmed cell death receptor 1 (PD1) inhibitor (budigalimab) to adult participants to treat NSCLC. ABBV-400 and budigalimab are investigational drugs being developed for the treatment of NSCLC. This study will be divided into two stages, with the first stage treating participants with several doses of ABBV-400 in combination with budigalimab within the dose escalation regimen until the dose reached is tolerable and expected to be efficacious. In Stage 2 there will be 4
Purpose
A Study to Evaluate the Adverse Events, Efficacy, and Optimal Dose of Intravenous (IV) Telisotuzumab Adizutecan in Combination With IV Budigalimab in Adult Participants With Advanced or Metastatic Non
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2025-03-06
Anticipated Date of Last Follow-up
2025-04-08
Estimated Primary Completion Date
2027-11-01
Estimated Completion Date
2027-11-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Must have histologically documented non-squamous (NSq) non small cell lung carcinoma (NSCLC) that is locally advanced or metastatic will be enrolled into the study. * Must have measurable disease per response evaluation criteria in solid tumors (RECIST) v1.1. * For Part 1, participants must have had no more than 1 systemic therapy for advanced disease including platinum-based chemotherapy or an immune checkpoint inhibitor (as monotherapy or in combination with chemotherapy), or appropriate targeted therapy for an actionable gene alteration, if applicable, for epidermal growth factor receptor (EGFR) wild-type (WT) NSq NSCLC. * For Part 2, participants must have no prior systemic therapy for advanced disease, no known actionable genomic alteration. * Must have document
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
172
Allocation
Randomized
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
TTX-030-003
Identifier
NCT06119217
Link
https://clinicaltrials.gov/study/NCT06119217
Phase
Phase II
Status
Not provided
Sponsor
Trishula Therapeutics, Inc.
More details
This is a Phase 2, multicenter, open-label, 3-arm, randomized, parallel group study to evaluate the efficacy and safety of TTX-030 with or without budigalimab in combination with chemotherapy (gemcitabine + nab-paclitaxel) in subjects with metastatic PDAC who did not have prior treatment for metastatic disease and are eligible to receive gemcitabine and nab-paclitaxel chemotherapy as SOC.
Purpose
Phase 2 Study of TTX-030 and Chemotherapy With or Without Budigalimab for 1L mPDAC Patients
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2024-03-25
Anticipated Date of Last Follow-up
2024-12-02
Estimated Primary Completion Date
2027-02-01
Estimated Completion Date
2027-06-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Abbreviated Inclusion Criteria: 1. Age 18 years or older, is willing and able to provide informed consent 2. Histologically or cytologically confirmed diagnosis of metastatic PDAC. 3. No prior systemic treatment for metastatic disease. 4. Evidence of measurable disease per RECIST 1.1. 5. Appropriate for treatment with nab-paclitaxel and gemcitabine chemotherapy. 6. Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1. Abbreviated Exclusion Criteria: 1. History of clinically significant allergy or hypersensitivity to planned study treatment components or to any monoclonal antibody 2. Use of investigational agent within 14 days prior to the first dose of study drug 3. History of autoimmune disease 4. Subject has received live vaccine within 28 days prior to the fir
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
194
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
M20-732
Identifier
NCT04807972
Link
https://clinicaltrials.gov/study/NCT04807972
Phase
Phase I
Status
Terminated
Sponsor
AbbVie
More details
Metastatic Pancreatic Cancer Disease is one of the most aggressive and deadliest forms of cancer with very poor survival. This study will evaluate adverse events and change in disease activity in participants 18 to 75 years of age with a body weight greater than or equal to 35 kg with Metastatic Pancreatic Cancer Disease treated with Intravenous (IV) infusion of modified FOLFIRINOX (mFFX) combined with IV infusions of ABBV-927 with or without Budigalimab. ABBV-927 and Budigalimab are the investigational drugs being developed for treatment of Metastatic Pancreatic Cancer Disease. In this study, doctors will enroll participants between 18 and 75 years of age with a body weight greater than or equal to 35 kg diagnosed diagnosed with Metastatic Pancreatic Cancer Disease in 4 different groups,
Purpose
Study to Evaluate Adverse Events and Change in Disease Activity When Intravenous (IV) Infusion of ABBV-927 is Administered in Combination With IV Modified FOLFIRINOX (mFFX) With or Without IV Budigali
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-05-28
Anticipated Date of Last Follow-up
2025-01-03
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2024-03-25
Actual Completion Date
2024-03-25
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Body weight \>= 35 kg. * Histologically or cytologically confirmed diagnosis of pancreatic adenocarcinoma with metastatic disease. * Measurable disease per Response Evaluation Criteria for Solid Tumors Version 1.1 (RECIST v1.1). * Prior history of or clinically stable concurrent malignancy are eligible for enrollment provided the malignancy is clinically insignificant, no treatment is required, and the participant is clinically stable. Exclusion Criteria: * Participants with locally advanced disease. * Participants with neuroendocrine (carcinoid, islet cell) or acinar pancreatic carcinoma. * Prior radiotherapy, surgery, or systemic anti-cancer therapy for the treatment of metastatic pancreatic adenocarcinoma. * Prior radiotherapy, surgery, or systemic anti-cancer t
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
40
Allocation
Randomized
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
Excipients
Proprietary excipients used
Not provided
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
Not provided
Residual solvents used
Not provided
Patent info
There are either no relevant patents or these were not yet submitted to LAPaL
Supporting material
Publications
Italiano, A., Cassier, P. A., Lin, C. C., Alanko, T., Peltola, K. J., Gazzah, A., Shiah, H. S., Calvo, E., Cervantes, A., Roda, D., Tosi, D., Gao, B., Millward, M., Warburton, L., Tanner, M., Englert, S., Lambert, S., Parikh, A., Afar, D. E., Vosganian, G., … Moreno, V. (2022). First-in-human phase 1 study of budigalimab, an anti-PD-1 inhibitor, in patients with non-small cell lung cancer and head and neck squamous cell carcinoma. Cancer immunology, immunotherapy : CII, 71(2), 417–431. https://doi.org/10.1007/s00262-021-02973-w
Abstract
Background: Budigalimab is a humanized, recombinant immunoglobulin G1 monoclonal antibody targeting programmed cell death protein 1 (PD-1). We present the safety, efficacy, pharmacokinetic (PK), and pharmacodynamic data from patients enrolled in the head and neck squamous cell carcinoma (HNSCC) and non-small cell lung cancer (NSCLC) expansion cohorts of the phase 1 first-in-human study of budigalimab monotherapy (NCT03000257; registered 15 December 2016).
Patients and methods: Patients with recurrent/metastatic HNSCC or locally advanced/metastatic NSCLC naive to PD-1/PD-1-ligand inhibitors were enrolled; patients were not selected on the basis of oncogene driver mutations or PD-L1 status. Budigalimab was administered at 250 mg intravenously Q2W or 500 mg intravenously Q4W until disease progression/unacceptable toxicity. The primary endpoints were safety and PK; the secondary endpoint was efficacy. Exploratory endpoints included biomarker assessments.
Results: In total, 81 patients were enrolled (HNSCC: N = 41 [PD-L1 positive: n = 19]; NSCLC: N = 40 [PD-L1 positive: n = 16]); median treatment duration was 72 days (range, 1-617) and 71 days (range, 1-490) for the HNSCC and NSCLC cohorts, respectively. The most frequent grade ≥ 3 treatment-emergent adverse event was anemia (HNSCC: n = 9, 22%; NSCLC: n = 5, 13%). Both dosing regimens had comparable drug exposure and increased interferon gamma-induced chemokines, monokine induced by gamma interferon, and interferon-gamma-inducible protein 10. Objective response rates were 13% (90% CI, 5.1-24.5) in the HNSCC cohort and 19% (90% CI, 9.2-32.6) in the NSCLC cohort. Median progression-free survival was 3.6 months (95% CI, 1.7-4.7) and 1.9 months (95% CI, 1.7-3.7) in the HNSCC and NSCLC cohorts.
Conclusions: The safety, efficacy and biomarker profiles of budigalimab are similar to other PD-1 inhibitors. Development of budigalimab in combination with novel anticancer agents is ongoing.
Calvo, E., Spira, A., Miguel, M., Kondo, S., Gazzah, A., Millward, M., Prenen, H., Rottey, S., Warburton, L., Alanko, T., Cassier, P. A., Yoh, K., Italiano, A., Moreno, V., Peltola, K., Seto, T., Toyozawa, R., Afar, D. E., Englert, S., Komarnitsky, P., … Gao, B. (2021). Safety, pharmacokinetics, and efficacy of budigalimab with rovalpituzumab tesirine in patients with small cell lung cancer. Cancer treatment and research communications, 28, 100405. https://doi.org/10.1016/j.ctarc.2021.100405
Abstract
Background: Agents targeting programmed cell death protein 1 (PD-1) have been approved as monotherapy for patients with small cell lung cancer (SCLC). In preclinical models, the combined targeting of PD-1 and delta-like protein 3 resulted in enhanced antitumor activity. Herein, we report results from the expansion arm of study NCT03000257 evaluating the combination of the anti-PD-1 antibody budigalimab and the targeted antibody-drug conjugate rovalpituzumab tesirine (Rova-T) in patients with previously treated SCLC.
Materials and methods: This expansion arm of a multicenter, open-label, multi-arm, first-in-human phase 1 clinical trial enrolled adult patients with progressive SCLC. The primary objective was to assess safety and tolerability. Patients received budigalimab 375 mg via intravenous infusion every 3 weeks, and Rova-T was administered as a dose of 0.3 mg/kg intravenously, on day 1 of the first and third 3-week cycle.
Results: As of October 2019, 31 patients with SCLC were enrolled and treated with budigalimab plus Rova-T. The combination was tolerated, with the most common treatment-emergent adverse events (in >30%) being pleural effusion, fatigue, and cough. The overall response rate was 24.1%, with one confirmed complete response and six confirmed partial responses. The overall response rate in patients with high delta-like protein 3 expression was similar (21.1%). The median progression-free survival was 3.48 months.
Conclusion: Combination therapy with budigalimab and Rova-T had promising efficacy and appeared to be tolerated in patients with SCLC. Although Rova-T development has been discontinued, development of budigalimab combined with other anticancer agents is ongoing.
Additional documents
No documents were uploaded
Useful links
There are no additional links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Not provided