
|
Developed by 

|
Supported by 

|
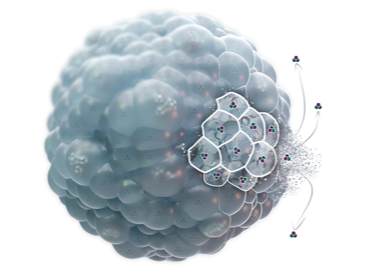
DepoFoam (Proprietary multivesicular liposome (pMVL) technology)
Based on public informationDeveloper(s)

|
Pacira Bioscience (formerly Skype Pharma Ltd) https://www.pacira.comUnited States of America Established in 2007, Pacira Biosciences has surfaced with a mission to furnish non-opioid therapy alternatives for individuals undergoing surgical procedures and experiencing acute pain. Their array of non-opioid injectables derives from their innovative liposomal technology. To amplify their pipeline, Pacira acquired Myoscience Ltd in 2019, fortifying their position in the pain management. |
Sponsor(s)
|
No sponsor indicated |
Partnerships

|
CrossLink Bioscience, LLC https://www.crosslinklifesciences.com/ |

|
Premier Inc. https://premierinc.com/ |
Technology information
Type of technology
Based on other organic particles
Administration route
Subcutaneous, Intramuscular, Intra-vitreal, Intravenous, Epidural, Intrathecal, Intraperitoneal
Development state and regulatory approval
Bupivacaine Hydrochloride
Marketed
Exparel has received approval from the United States Food and Drug Administration and marketing authorization from the European Medicines Agency. It is indicated for post-surgical analgesia.
Description
DepoFoam is a lipid-based sustained-release drug delivery system that encapsulates the API into an interconnected network of multi-layered multivesicular liposomes (MVL). These liposomes are highly stable and release the drug from the outermost member of the MVL into the external medium. Exhibiting a microscopic, non-concentric structure, these lipid-based spheroids harbor granular lipid-based particles housing the encapsulated API. The neutral lipid component comprises mono-unsaturated fatty acid ester moieties containing approximately 14–18 carbons, primarily in the form of triglycerides.
Technology highlight
• The release rate of the API can be modified based on the preparation of the MVL and the neutral lipid component added in the formulation. • The Multivesicular liposomes are biodegradable in nature and biocompatibility for targeted drug delivery at sensitive areas like ocular, epidural, and intrathecal routes of administration. • Optimal concentration at the targeted anatomical location with low systematic exposure.
Technology main components
The pMVL formulation contains multi-vesicular liposomes, dextrose, triolein & tricaprylin (mole ratio based on the characteristics of API), phospholipids, DEPC, L-lysine, & DPPG.
Information on the raw materials sourcing, availability and anticipated price
The DPPG, triolein, and tricaprylin are obtained from Avanti Polar Lipids, and 10% hydrochloric acid is obtained from Spectrum Chemical. The current cost of the FDA-approved pMVL product Exparel 133 mg (10 mL) dose is 227.63 USD, and Exparel 266 mg (20 mL) dose is 376.12 USD.
Delivery device(s)
No delivery device
APIs compatibility profile
API desired features
Water-soluble molecules
Water-insoluble molecules
Small molecules
Small molecules in therapeutic areas such as antitumor agents, anaesthetics, analgesics, antimicrobial agents, opiates, hormones etc are considered to be the main choice of interest for pMVL formulation.
Nucleic acids
Nucleic acids, including DNA, RNA, and antisense oligonucleotides, can be encapsulated using pMVL, and they are selected on a case-by-case basis.
Proteins
Proteins and peptides, and other compounds like cytokines, hormones (pituitary and hypophyseal hormones), growth factors, vaccines can be encapsulated in the pMVL matrix. Of particular interest are interleukin-2, insulin-like growth factor-1, interferons, insulin, heparin, leuprolide, granulocyte colony Stimulating factor (G-CSF), granulocyte-macrophage colony stimulating factor (GM-CSF), tumor necrosis factor, inhibin, tumor growth factor alpha and beta, Mullerian inhibitory substances, calcitonin, and hepatitis B vaccine.
Additional solubility data
Not provided
Additional stability data
Not provided
API loading: Maximum drug quantity to be loaded
≥ 80%
API co-administration
Not provided
LogP
Not provided
Scale-up and manufacturing prospects
Scale-up prospects
The company has expanded to a 200-liter manufacturing suite, which has the capacity to produce up to 200 million doses per year.
Tentative equipment list for manufacturing
Nozzle valve type spray dryer, solvent removal vessel, a solvent removal gas exit orifice comprising a gas outlet tube, three-fluid atomizing nozzle apparatus, continuous-flow emulsification system, continuous processing system, evaporation apparatus, high shear mixer.
Manufacturing
• GMP ISO 7 or higher • First Emulsion - API lipid emulsion is formulated volatile water - immiscible solvent and filtered in aseptic environment • H3PO4 solution is made, filtered and mixed with first emulsion to produce w/o emulsion • Second Emulsion - Dextrose Lysin Solution is made, filtered and w/o emulsion is added to this solution to produce w/o/w emulsion is produced • Solvent Extraction - Sparge the prepared w/o/w emulsion in the Sparge Vessel and extract the concentrate • Microfiltration - Microfiltration using Diafiltration vessel via NaCl in a cross-flow filtration system.
Specific analytical instrument required for characterization of formulation
• Freeze-fracture electron microscopy • 31P NMR spectroscopy, confocal microscopy to identify the locations of triglycerides in the MVL matrix. • HPLC to identify impurities in the lipid matrix.
Clinical trials
402-C-125
Identifier
NCT06271265
Link
https://clinicaltrials.gov/study/NCT06271265
Phase
Phase I
Status
Recruiting
Sponsor
Pacira Pharmaceuticals, Inc
More details
This Phase 1, multicenter, open-label, randomized, bupivacaine-controlled study is designed to evaluate the pharmacokinetics (PK) and safety of EXPAREL vs. bupivacaine HCl for postsurgical analgesia in pediatric subjects aged 0 to less than 6 years of age undergoing cardiac surgery, utilizing local infiltration analgesia (LIA).
Purpose
Study to Evaluate the Pharmacokinetics and Safety of EXPAREL for Postoperative Analgesia in Subjects Undergoing Cardiac Surgery
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2024-03-29
Anticipated Date of Last Follow-up
2024-06-04
Estimated Primary Completion Date
2025-07-01
Estimated Completion Date
2025-07-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
Unspecified
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Parent/guardian able to speak, read, and understand the language of the ICF and provide informed consent for the subject. 2. American Society of Anesthesiologists (ASA) Class 1-3. 3. Male or female subjects from 0 to less than 6 years of age on the day of surgery. For Part 1, the subject's age should be 2 years to less than 6 years. For Part 2, the subject's age should be 6 months to less than 2 years. For Part 3, the subject's age should be 0 to less than 6 months. 4. Parent/guardian able to adhere to the study visit schedule and complete all study assessments for the subject.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
48
Allocation
Randomized
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
| Type | Title | Content | Link |
|---|---|---|---|
| Link | Study to Evaluate the Pharmacokinetics and Safety of EXPAREL for Postoperative Analgesia in Subjects Undergoing Cardiac Surgery | https://clinicaltrials.gov/study/NCT06271265 |
402-C-122
Identifier
NCT04002089
Link
https://clinicaltrials.gov/study/NCT04002089
Phase
Phase I
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
This is a pilot, open label, single center study in 40 subjects undergoing bunionectomy. The study will assess and collect information on pharmacokinetics, pharmacodynamics, safety and efficacy of EXPAREL administered as a sciatic nerve block (in popliteal fossa). A total of 10 subjects will be enrolled in each of the 4 cohorts.
Purpose
Study to Evaluate the Pharmacokinetics, Pharmacodynamics and Safety, of EXPAREL Administered as Sciatic Nerve Block (In Popliteal Fossa), for Postsurgical Analgesia in Subjects Undergoing Bunionectomy
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-07-26
Anticipated Date of Last Follow-up
2021-01-07
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2019-12-02
Actual Completion Date
2019-12-02
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Healthy adult male or female volunteers ages 18 or older 2. American Society of Anesthesiologists (ASA) physical status 1, 2, or 3 3. Able to provide informed consent, adhere to the study schedule, and complete all study assessments. 4. Body Mass Index ≥18 and ≤40 kg/m2.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
45
Allocation
Non-randomized
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
402-C-117
Identifier
NCT02985762
Link
https://clinicaltrials.gov/study/NCT02985762
Phase
Phase I
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
The primary objective of this study is to characterize the pharmacokinetic (PK) profile of EXPAREL when administered via local wound infiltration to subjects undergoing open spinal fusion or reconstructive surgery. The secondary objectives of this study are to assess the safety, tolerability, and efficacy of EXPAREL in this surgical model.
Purpose
PK Study of EXPAREL in Subjects Undergoing Open Spinal Fusion or Reconstructive Surgery
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2016-12-01
Anticipated Date of Last Follow-up
2018-04-09
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2017-09-01
Actual Completion Date
2017-09-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Males or females ≥18 years of age. 2. American Society of Anesthesiologists (ASA) physical status 1, 2, or 3. Scheduled to undergo primary, ≥3 level cervical or thoracic spine fusion or reconstruction under general anesthesia. The surgical incision must be at least 8 cm in length. 3. Female subject must be surgically sterile; or at least 2 years postmenopausal; or have a monogamous partner who is surgically sterile. 4. Able to provide informed consent.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
15
Allocation
Non-randomized
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
402-C-120
Identifier
NCT03485014
Link
https://clinicaltrials.gov/study/NCT03485014
Phase
Phase I
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
Primary objective: The primary objective of this study is to evaluate the pharmacokinetics (PK) of EXPAREL in pediatric subjects 12 to less than 17 years of age undergoing spinal surgery. Secondary objective: The secondary objective of this study is to evaluate the safety of EXPAREL in pediatric subjects 12 to less than 17 years of age undergoing spinal surgery.
Purpose
Study in Pediatric Subjects Evaluating Pharmacokinetics and Safety of EXPAREL
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2018-04-10
Anticipated Date of Last Follow-up
2023-08-01
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2019-02-12
Actual Completion Date
2019-02-12
Studied populations
Age Cohort
- Children
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: 1. Subjects whose parent(s) or guardian(s) has/have signed and dated an informed consent form for the subject to participate in the study. 2. American Society of Anesthesiologists (ASA) Class 1-3. 3. Male or female subjects 12 to less than 17 years of age on the day of surgery. 4. Body mass index (BMI) at screening within the 20th to 80th percentile for age and sex. 5. A pregnancy test for female subjects of childbearing potential will be conducted in the preoperative holding area according to the study site's standard of care. A negative result for the pregnancy test must be available prior to the start of surgery. 6. Subjects and their parent(s)/guardian(s) must be able to speak. 7. Subjects must be able to adhere to the study visit schedule .
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
15
Allocation
Non-randomized
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
EXPAREL Dose-Response
Identifier
NCT01349140
Link
https://clinicaltrials.gov/study/NCT01349140
Phase
Phase I
Status
Completed
Sponsor
University of California, San Diego
More details
EXPAREL™, an investigational drug product, is a new formulation of a local anesthetic (numbing medicine) that is designed to be longer acting than the currently-available local anesthetics. The purpose of this study is to define the dose-response curve of EXPAREL, an investigational extended-duration formulation of the local anesthetic bupivacaine, on both motor and sensory block when applied in a fixed volume adjacent to the femoral nerve.
Purpose
EXPAREL Dose-Response for Single-Injection Femoral Nerve Blocks
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2012-02-01
Anticipated Date of Last Follow-up
2021-03-23
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2012-04-01
Actual Completion Date
2012-04-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: * greater than or equal to 18 years old * able and willing to have bilateral femoral nerve blocks placed and repeated motor/sensory testing for 24-120 hours (1-5 days), requiring 1-5 overnight stay(s) in the UCSD CTRI to allow dissipation of local anesthetic infusion effects to near-baseline values * have the ability to adequately communicate with all study personnel * willing and capable of providing written informed consent. Exclusion Criteria: * daily analgesic use for over one week within the past 6 months * opioid use within the previous 4 weeks * any neuro-muscular deficit of either femoral nerves and/or quadriceps muscles * body mass index \> 30 kg/m2 * current pregnancy * incarceration * any coagulation disorder * uncontrolled anxiety, schizophrenia, or other
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
14
Allocation
Randomized
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Double-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
402-C-123
Identifier
NCT04293809
Link
https://clinicaltrials.gov/study/NCT04293809
Phase
Phase I
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
This is a pilot, open label, single center study in 30 women undergoing breast augmentation. The study will assess and collect information on pharmacokinetics and safety of EXPAREL administered as a pectoral plane block. A total of 15 subjects will be enrolled in each of the 2 cohorts.
Purpose
Study to Evaluate the Pharmacokinetics and Safety of EXPAREL Administered as a Pectoral Plane Block in Women Undergoing Breast Augmentation
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-12-19
Anticipated Date of Last Follow-up
2024-01-26
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2020-01-15
Actual Completion Date
2020-01-29
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Female
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Women aged 18 or older, undergoing breast augmentation and are American Society of Anesthesiologists (ASA) physical status 1, 2, or 3 2. Able to provide informed consent, adhere to the study schedule, and complete all study assessments 3. Body Mass Index ≥18 and ≤30 kg/m2 Exclusion Criteria: 1. Allergy, hypersensitivity, intolerance, or contraindication to any of the study medications for which an alternative is not named in the protocol (e.g., amide-type local anesthetics, opioids, bupivacaine, NSAIDs) 2. Documented history of long-term diabetes (≥10 years) or severe peripheral vascular disease 3. Renal (serum creatinine level \>2mg/dL \[176.8 μmol/L\]) or hepatic dysfunction (serum alanine or aspartame transferase \> 3 times the upper limit of normal) 4. Concurr
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
30
Allocation
Randomized
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
402-C-113
Identifier
NCT02210247
Link
https://clinicaltrials.gov/study/NCT02210247
Phase
Phase I
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
The purpose of this study is to evaluate the safety and pharmacokinetics of redosing EXPAREL via local subcutaneous infiltration in healthy volunteers.
Purpose
Safety and Pharmacokinetics Study of Redosing EXPAREL in Healthy Volunteers
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2014-08-01
Anticipated Date of Last Follow-up
2021-07-01
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2014-09-01
Actual Completion Date
2014-09-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: * Males or females ≥18 years of age. * American Society of Anesthesiologists (ASA) physical status 1 or 2. * Female subjects must be surgically sterile, at least 2 years postmenopausal, or using a medically acceptable method of birth control. If of childbearing potential, must have a documented negative pregnancy test within 24 hours before the first study drug administration. * Able to provide informed consent, adhere to the study visit schedule, and complete all study assessments.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
60
Allocation
Not provided
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Not provided
Studied LA-formulation(s)
Not provided
Studied route(s) of administration
Not provided
Use case
Not provided
Key resources
SKY0402C209
Identifier
NCT00529126
Link
https://clinicaltrials.gov/study/NCT00529126
Phase
Phase II
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
Phase 2 study to evaluate three dose levels of SKY0402 compared with 75 mg of bupivacaine HCl.
Purpose
Phase 2 Dose-Ranging Study of SKY0402 for Prolonged Postoperative Analgesia in Subject Undergoing Hemorrhoidectomy
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2007-09-01
Anticipated Date of Last Follow-up
2021-02-11
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2007-12-01
Actual Completion Date
2008-07-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Male or female, 18 years of age and older at the Screening Visit. 2. Applies to female subjects only: Postmenopausal, surgically sterile, or willing to use acceptable means of contraception for at least 30 days after surgery including any of the following: hormonal contraceptives (e.g., oral, injectable, implantable starting at least 30 days before study drug administration), effective double-barrier methods (e.g., condoms with spermicide), intrauterine device, lifestyle with a personal choice of abstinence, nonheterosexual lifestyle, or a strictly monogamous relationship with a partner who has had a vasectomy. 3. Scheduled to undergo 2- or 3-column excisional hemorrhoidectomy under general anesthesia.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
100
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
TKA
Identifier
NCT00485693
Link
https://clinicaltrials.gov/study/NCT00485693
Phase
Phase II
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
Dose-ranging study for prolonged postoperative analgesia in subjects undergoing total knee arthroplasty
Purpose
Dose-Ranging Study for Prolonged Postoperative Analgesia in Subject Undergoing Total Knee Arthroplasty
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2007-06-01
Anticipated Date of Last Follow-up
2022-06-07
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2009-08-01
Actual Completion Date
2009-08-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Male or female, greater than or equal to 18 and less than or equal to 75 years of age at the Screening Visit. 2. Female subjects only: Postmenopausal, surgically sterile, or willing to use acceptable means of contraception for at least 30 days after surgery. 3. Scheduled to undergo primary unilateral total knee arthroplasty (TKA) under general anesthesia. 4. American Society of Anesthesiology (ASA) Physical Class 1-3.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
138
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Double-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
SKY0402-C-207
Identifier
NCT00485433
Link
https://clinicaltrials.gov/study/NCT00485433
Phase
Phase II
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
The purpose of this study is to evaluate three dose levels of SKY0402 compared with 105 mg of bupivacaine HCl.
Purpose
Dose-ranging Study for Postoperative Analgesia in Subjects Undergoing Primary Unilateral Inguinal Hernia Repair
Interventions
Intervention 1
Intervention 2
Countries
Not provided
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2007-06-01
Anticipated Date of Last Follow-up
2021-02-11
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2007-12-01
Actual Completion Date
2008-08-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Male
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Males 18 years of age and older at the Screening Visit. 2. Scheduled to undergo, elective, primary, unilateral, open-technique, tension-free (Lichtenstein-type technique with mesh) inguinal hernia repair under general anesthesia. 3. American Society of Anesthesiology (ASA) Physical Class 1-3. 4. Capable and willing to comply with all study visits and procedures and to provide written informed consent. 5. Able to speak, read, and understand the language of the informed consent form, study questionnaires, and other instruments used for collecting subject-reported outcomes, in order to enable accurate and appropriate responses to pain scales and other required study assessments. Exclusion Criteria: 1. Use of any of the following medications within the times specified
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
98
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
1602
Identifier
NCT03106545
Link
https://clinicaltrials.gov/study/NCT03106545
Phase
Phase II
Status
Completed
Sponsor
Catherine Vandepitte, M.D.
More details
Postoperative analgesia for hallux valgus surgery (bunionectomy) is inconsistent and may even result in rebound pain when the (ankle) blocks wear off. It is hypothesized that the mixture of bupivacaine and liposome bupivacaine increases the extent and duration of postoperative analgesia and decreases opioid consumption as compared to bupivacaine alone or to general anesthesia.
Purpose
Liposome Bupivacaine for ANKLE Blocks
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2017-01-02
Anticipated Date of Last Follow-up
2020-09-09
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2017-06-26
Actual Completion Date
2017-10-31
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female, at least 18 years and max 65 years of age at screening * Scheduled to undergo primary Scarf osteotomy for elective hallux valgus * American Society of Anesthesiologists (ASA) physical status 1, 2, or 3 * Female subject must be surgically sterile or have a monogamous partner who is surgically sterile. * Able to demonstrate sensory function by exhibiting sensitivity to cold, pinprick, and light touch.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
40
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
ETSP
Identifier
NCT03739021
Link
https://clinicaltrials.gov/study/NCT03739021
Phase
Phase II/III
Status
Not yet recruiting
Sponsor
University of South Florida
More details
Total shoulder surgery (arthroplasty) is a widely successful method of treating shoulder arthritis. Although the goal of the procedure is pain relief, post-operative pain is unavoidable. Pain is a common side effect that many patients undergo while in the clinical setting and is a vital factor in influencing the length of hospital stay, narcotic usage, as well as overall patient satisfaction. Post-operative pain management typically involves elevated usage of narcotics, which is a concern among clinicians and researchers alike. To combat this issue, research is examining intraoperative procedures as a means of reducing post-operative pain scores. Research has discovered the advantages of utilizing local anesthetic techniques as opposed to just general anesthesia. Local anesthetic blocks f
Purpose
Exparel for Total Shoulder Pain
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2019-11-01
Actual Start Date
Not provided
Anticipated Date of Last Follow-up
2019-07-15
Estimated Primary Completion Date
2020-03-01
Estimated Completion Date
2020-11-15
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Patients 18 years or older * Patients admitted to TGH for total shoulder replacement surgery * Subjects who have given written informed consent. Exclusion Criteria: * Patients with allergic reactions to Exparel or Ropivacaine * Female patients who are pregnant * Subjects known or suspected to have neuromuscular disorders impairing neuromuscular blockade (e.g., subjects with myasthenia gravis)
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
60
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
Pro2022001580
Identifier
NCT06077422
Link
https://clinicaltrials.gov/study/NCT06077422
Phase
Phase II/III
Status
Recruiting
Sponsor
Rutgers, The State University of New Jersey
More details
The goal of this pilot study is to describe and compare Ultrasound-Guided Erector Spinae Plane (ESP) Blocks using Exparel® (bupivacaine liposome injectable suspension) to Marcaine® (bupivacaine hydrochloride) for pain management and outcomes after cardiac surgeries.
Purpose
Exparel vs. Marcaine ESP Block for Post-cardiac Surgical Pain
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2024-01-11
Anticipated Date of Last Follow-up
2024-01-24
Estimated Primary Completion Date
2025-01-11
Estimated Completion Date
2025-04-05
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Adults (18 years-no upper age limit) * Scheduled for mini thoracotomy (i.e. valve repair) or open sternotomy (i.e. bypass graft) at single academic medical center (in and out-patients). Exclusion Criteria: Patients will be excluded if they: * Are currently on pain medication or pain regimen for chronic pain condition * Convert to sternotomy (for thoracotomies) * Require, upon intraoperative discovery and surgeon's decision, the need for an unplanned secondary procedure other than the originally scheduled index operation * Undergo emergent surgery * Are non-English speaking (The majority of the PI's patient population speak English. As a pilot study the investigators cannot afford to enroll non-English speaking subjects due to time, personnel, and financial constra
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
120
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Not provided
Studied route(s) of administration
Use case
PEP
Key resources
402-C-323
Identifier
NCT01683071
Link
https://clinicaltrials.gov/study/NCT01683071
Phase
Phase II/III
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
The primary objectives of Part 1 are to (1) evaluate three dose levels of liposome bupivacaine versus placebo with respect to the magnitude and duration of the analgesic effect achieved following single dose injection femoral nerve block with liposome bupivacaine, and (2) select a single therapeutic dose of liposome bupivacaine from the three dose levels to be tested in Part 2. Part 2: The primary objective of Part 2 is to compare the magnitude and duration of the analgesic effect of single injection femoral nerve block of a single dose level of liposome bupivacaine (selected from Part 1) with placebo (preservative-free normal saline).
Purpose
Femoral Nerve Block With Liposome Bupivacaine for Postsurgical Analgesia Following Total Knee Arthroplasty
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2012-09-01
Anticipated Date of Last Follow-up
2020-11-15
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2013-12-01
Actual Completion Date
2013-12-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Male or female, \>=18 years of age. 2. Scheduled to undergo primary unilateral TKA under general or spinal anesthesia. 3. American Society of Anesthesiologists (ASA) Physical Status 1, 2, or 3. 4. Able to demonstrate motor function by performing a 20-meter walk, unassisted with the optional use of a 4-legged walker, and sensory function by exhibiting sensitivity to cold. 5. Able to provide informed consent, adhere to the study visit schedule, and complete all study assessments. Exclusion Criteria: 1. Currently pregnant, nursing, or planning to become pregnant during the study or within 1 month after study drug administration. Female subjects must be surgically sterile, at least 2 years menopausal, or using an acceptable method of birth control.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
297
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
STU00214187
Identifier
NCT05171179
Link
https://clinicaltrials.gov/study/NCT05171179
Phase
Phase III
Status
Recruiting
Sponsor
Northwestern University
More details
This project intends to more thoroughly investigate the direct influence of Pecs blocks in the administration of Exparel, a non-opioid analgesic, in breast reconstruction surgery. The hypothesis is that this analgesic delivery method will significantly reduce negative outcomes such as post-operative pain, opioid use, and nausea while increasing positive outcomes such as post-operative physical activity.
Purpose
The Use of Pecs Blocks in Combination With Exparel in Breast Reconstruction Surgery
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-10-22
Anticipated Date of Last Follow-up
2023-11-08
Estimated Primary Completion Date
2024-04-01
Estimated Completion Date
2024-04-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Female
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: 1. Subjects greater than 18 years of age. 2. Subject who are undergoing implant-based, tissue expander breast reconstruction surgery. Exclusion Criteria: 1. Subjects undergoing flap breast reconstruction. 2. Subjects who are undergoing direct-to-implant surgery. 3. Subjects who have previously undergone radiation therapy. 4. Medical or psychiatric condition that may increase the risk associated with study participation, may complicate patient compliance, or may interfere with the interpretation of study results and, in the judgment of the Investigator, would make the subject inappropriate for entry into this study. 5. Subjects who are pregnant at the date of surgery.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
90
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Single blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
PLAY
Identifier
NCT03682302
Link
https://clinicaltrials.gov/study/NCT03682302
Phase
Phase III
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
Primary Objective: To evaluate the pharmacokinetics (PK) of EXPAREL in pediatric subjects aged 6 to less than 17 years undergoing various types of surgeries. Secondary Objective: To evaluate the safety of EXPAREL in pediatric subjects aged 6 to less than 17 years undergoing various types of surgeries.
Purpose
Multicenter Study for Pediatric Subjects Evaluating Pharmacokinetics and Safety of EXPAREL
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-04-02
Anticipated Date of Last Follow-up
2020-12-10
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2019-08-30
Actual Completion Date
2019-09-24
Studied populations
Age Cohort
- Children
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Subjects whose parent(s) or guardian(s) has/have signed and dated the ICF for the subject to participate in the study, and subjects who have provided written assent to participate in the study (if capable). 2. American Society of Anesthesiologists (ASA) Class 1-3. 3. Male or female subjects 6 to less than 17 years of age on the day of surgery. 4. Body mass index (BMI) at screening within the 5th to 95th percentile for age and sex (see Appendix 5). 5. A negative pregnancy test for female subjects of childbearing potential must be available prior to the start of surgery. The pregnancy test must be conducted in the preoperative holding area according to the study site's standard of care.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
98
Allocation
Randomized
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
SKY0402C316
Identifier
NCT00890721
Link
https://clinicaltrials.gov/study/NCT00890721
Phase
Phase III
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
Patients will get an injection of either SKY0402 or placebo during hemorrhoid surgery, and their pain and pain medicine use will be monitored.
Purpose
Study of Pain Control in Hemorrhoidectomy
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2009-05-01
Anticipated Date of Last Follow-up
2013-07-03
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2009-11-01
Actual Completion Date
2009-11-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Age \> 18 years of age at the Screening visit * American Society of Anesthesiologists (ASA) class 1-3 * Scheduled to have a two or three column excisional hemorrhoidectomy under general anesthesia using the Milligan-Morgan technique * For female subjects, surgically sterile or at least two years menopausal, or using an acceptable method of birth control; must have a documented negative blood or urine pregnancy test within 24 hours before surgery * Clinical lab values less than or equal to twice the upper limit of normal or, if abnormal, deemed not clinically significant per the Investigator. * Ability to understand the requirements of the study, provide written informed consent, abide by the study restrictions, and agree to return for the required assessments.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
189
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Double-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
PRO17060305
Identifier
NCT03270514
Link
https://clinicaltrials.gov/study/NCT03270514
Phase
Phase III
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
The aim of this study is to evaluate the analgesic efficacy and safety of wound infiltration with liposomal bupivacaine (LB) in patients undergoing cardiac surgery with sternotomy and cardiopulmonary bypass (CPB) and compare it with bupivacaine hydrochloride infiltration
Purpose
Comparison of Sternal Wound Infiltration With Liposomal Bupivacaine v. Bupivacaine Hydrochloride
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2018-11-15
Anticipated Date of Last Follow-up
2021-08-10
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2019-12-13
Actual Completion Date
2020-02-25
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Open cardiac surgery through sternotomy approach (eg. coronary artery bypass graft, valvular heart procedures, as well as other open cardiac procedures along with coronary artery bypass) * Surgery with the use of cardiopulmonary bypass Exclusion Criteria: * Minimally invasive heart surgery through thoracotomy approach * Patient undergoing procedures under deep hypothermic circulatory arrest * Patients with active infections such as infective endocarditis * Emergency surgery * Patients undergoing transplantations and ventricular assist device insertion * Patients on any mechanical circulatory support preoperatively * Patient's refusal * End stage liver or renal disease * Allergy to bupivacaine * Patient who cannot understand the study procedure or refuse to particip
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
60
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Not provided
Studied LA-formulation(s)
Not provided
Studied route(s) of administration
Not provided
Use case
Not provided
Key resources
SKY0402C317
Identifier
NCT00890682
Link
https://clinicaltrials.gov/study/NCT00890682
Phase
Phase III
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
After undergoing bunion surgery, patients are given a pain medicine injection that may last for up to several days or a placebo. Their pain and pain medicine use is then monitored.
Purpose
Study of Postoperative Analgesia in Bunionectomy
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2009-04-01
Anticipated Date of Last Follow-up
2013-07-02
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2009-11-01
Actual Completion Date
2009-11-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Age ≥ 18 years at the Screening visit * Scheduled to undergo primary unilateral first metatarsal osteotomy without hammertoe * Female subjects must be surgically sterile or at least two years menopausal, or using an acceptable method of birth control. If of childbearing potential, have a documented negative blood or urine pregnancy test within 24 hours before surgery * Clinical laboratory values less than or equal to twice the upper limit of normal or, if abnormal, deemed not clinically significant per the Investigator * Ability to provide informed consent, adhere to the study visit schedule and complete all study assessments Exclusion Criteria: * Currently pregnant, nursing, or planning to become pregnant during the study or within one month after giving the drug.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
193
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Double-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
J17157
Identifier
NCT04117074
Link
https://clinicaltrials.gov/study/NCT04117074
Phase
Phase III
Status
Recruiting
Sponsor
Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins
More details
The goal of this study is to test the hypothesis that surgical site infiltration with liposomal bupivacaine (LB) is non-inferior to and more cost effective than thoracic epidural analgesia (TEA) for patients undergoing open gynecologic surgery on an established enhanced recovery program (ERP) using a non-inferiority randomized trial design. The impact of TEA and surgical site infiltration with LB on neuroendocrine and inflammatory mediators of surgical stress response (SSR) will also be investigated as a translational endpoint.
Purpose
Thoracic Epidural Analgesia vs Surgical Site Infiltration With Liposomal Bupivacaine Following Open Gynecologic Surgery
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-04-14
Anticipated Date of Last Follow-up
2023-06-16
Estimated Primary Completion Date
2024-09-01
Estimated Completion Date
2025-09-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Female
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: * Individuals ≥ 18 years of age * Planned laparotomy by the gynecologic oncology service at the sponsor institution. Exclusion Criteria: * Individuals who have a contraindication to thoracic epidural analgesia * Individuals with a coagulation disorder * Individuals with an infection at the site of epidural placement * Individuals with intracranial pathology such as non-communicating increased intracranial pressure or obstruction of cerebrospinal fluid flow related to mass lesions * Individuals with spinal pathology: abnormal spine anatomy, surgical fusion, or spinal column lesions * Individuals who have a contraindication to liposomal bupivacaine * Individuals with a known allergic reaction to liposomal bupivacaine * Individuals with Childs-Pugh Class B or C.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
100
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
STRIDE
Identifier
NCT04518462
Link
https://clinicaltrials.gov/study/NCT04518462
Phase
Phase III
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
This is a Phase 3, multicenter, randomized, double blinded, active controlled study in approximately 120 subjects undergoing lower extremity surgeries.
Purpose
Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of EXPAREL, EXPAREL Admixed With Bupivacaine HCl vs. Bupivacaine HCl Administered as Combined Sciatic and Saphenous Nerve Blocks for Postsu
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2020-10-20
Anticipated Date of Last Follow-up
2022-07-15
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2021-04-05
Actual Completion Date
2021-04-05
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Healthy adult male or female volunteers ages 18 or older 2. American Society of Anesthesiologists (ASA) physical status 1, 2, or 3 3. Able to provide informed consent, adhere to the study schedule, and complete all study assessments 4. Body Mass Index (BMI) ≥18 and ≤40 kg/m2 Exclusion Criteria: 1. Allergy, hypersensitivity, intolerance, or contraindication to any of the study medications for which an alternative is not named in the protocol (e.g., amide-type local anesthetics, opioids, bupivacaine HCl, nonsteroidal anti-inflammatory drugs \[NSAIDs\]) 2. Concurrent painful physical condition that may require analgesic treatment in the post dosing period for pain.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
121
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
402-C-327
Identifier
NCT02713230
Link
https://clinicaltrials.gov/study/NCT02713230
Phase
Phase III
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
This is a Phase 3, multicenter, randomized, double-blind, placebo-controlled study in 155 adult subjects undergoing primary unilateral total shoulder arthroplasty or rotator cuff repair with general anesthesia
Purpose
Efficacy, Safety, and Pharmacokinetics of Brachial Plexus Block With EXPAREL in Shoulder Surgery
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2016-05-09
Anticipated Date of Last Follow-up
2020-11-06
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2017-07-07
Actual Completion Date
2017-07-07
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Male or female, at least 18 years of age at screening. 2. Scheduled to undergo primary unilateral total shoulder arthroplasty or rotator cuff repair. 3. Subjects scheduled for rotator cuff repair must have a magnetic resonance imaging (MRI) with a reading confirming a tear of at least 1 cm. 4. American Society of Anesthesiologists (ASA) physical status 1, 2, or 3. 5. Female subject must be surgically sterile; or at least 2 years postmenopausal; or have a monogamous partner who is surgically sterile; or practicing double-barrier contraception; or practicing abstinence (must agree to use double-barrier contraception in the event of sexual activity); or using an insertable, injectable, transdermal, or combination oral contraceptive approved by the FDA for greater than
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
156
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
402-C-335
Identifier
NCT05139030
Link
https://clinicaltrials.gov/study/NCT05139030
Phase
Phase III
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
The purpose of the study is to compare magnitude of postsurgical analgesic effect in different groups following a single dose of study drug when administered via adductor canal block in subjects undergoing primary unilateral total knee arthroplasty.
Purpose
Phase 3 Adductor Canal Block With EXPAREL in Subjects Undergoing Primary Unilateral Total Knee Arthroplasty
Interventions
Intervention 1
Intervention 2
Countries
Not provided
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-01-18
Anticipated Date of Last Follow-up
2022-09-06
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-07-01
Actual Completion Date
2022-07-11
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Male or female, ages 18 or older at screening. 2. Indicated to undergo primary unilateral total knee arthroplasty under spinal anesthesia. 3. Primary indication for TKA is degenerative osteoarthritis of the knee. 4. American Society of Anesthesiologists (ASA) physical status 1, 2, or 3. 5. Able to provide informed consent, adhere to the study schedule, and complete all study assessments. 6. Body Mass Index (BMI) ≥18 and \<40 kg/m2. Exclusion Criteria: 1. Allergy, hypersensitivity, intolerance, or contraindication to any of the study medications for which an alternative is not named in the protocol (e.g., amide-type local anesthetics, opioids, bupivacaine HCl, NSAIDs). 2. Planned concurrent surgical procedure (e.g., bilateral TKA). 3. Undergoing unicompartmental TK
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
167
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
402-C-334
Identifier
NCT05157841
Link
https://clinicaltrials.gov/study/NCT05157841
Phase
Phase III
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
The study is conducted sequentially in two parts. Part A: The purpose is to obtain information on PK profile, pharmacodynamics (PD), efficacy, safety, and to assess the performance of 266 mg EXPAREL vs 133 mg EXPAREL. Part B: The purpose is to evaluate the efficacy and safety of the preferred dosage of EXPAREL from Part A compared with bupivacaine HCl.
Purpose
Phase 3, Sciatic Nerve Block With EXPAREL for Subjects Undergoing Bunionectomy
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-02-10
Anticipated Date of Last Follow-up
2022-09-06
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-08-07
Actual Completion Date
2022-08-15
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Male or female, ages 18 or older at screening 2. American Society of Anesthesiologists (ASA) physical status 1, 2, or 3 3. Able to provide informed consent, adhere to the study schedule, and complete all study assessments 4. Primary surgical indication is related to a bunion deformity (i.e., hallux valgus) and subject is scheduled to undergo a distal metaphyseal osteotomy procedure (e.g., Austin procedure as opposed to Lapiplasty, Lapidus bunionectomies or base wedge bunionectomies) 5. Indicated to undergo elective (i.e., not emergency) bunionectomy 6. Body Mass Index (BMI) ≥18 and \<40 kg/m2 Exclusion Criteria: 1. Allergy, hypersensitivity, intolerance, or contraindication to any of the study medications for which an alternative is not named in the protocol.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
185
Allocation
Randomized
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
402-C-329
Identifier
NCT02517905
Link
https://clinicaltrials.gov/study/NCT02517905
Phase
Phase III
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
This is a Phase 3, randomized, double-blind, placebo-controlled study in subjects scheduled to undergo elective bilateral third molar extraction under local anesthesia. At least one lower mandibular third molar must involve full or partial bony impaction confirmed by visual or radiographic evidence.
Purpose
Evaluation of EXPAREL for Prolonged Postsurgical Analgesia in Subjects Undergoing Third Molar Extraction
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2015-08-01
Anticipated Date of Last Follow-up
2020-11-15
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2016-01-01
Actual Completion Date
2016-01-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Male or female, ≥18 years of age at screening. 2. Scheduled to undergo bilateral third molar extractions (i.e., extraction of all four third molars) under local anesthesia. At least one lower mandibular third molar must involve full or partial bony impaction confirmed by visual or radiological evidence. 3. American Society of Anesthesiology (ASA) physical status 1, 2, or 3. 4. Female subjects must be either surgically sterile, using a medically acceptable method of birth control, or at least 2 years postmenopausal, and must have a documented negative pregnancy test result during screening and on Day 1 prior to surgery. 5. Able to provide informed consent, adhere to the study visit schedule, and complete all study assessments.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
166
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
402-C-322
Identifier
NCT01802411
Link
https://clinicaltrials.gov/study/NCT01802411
Phase
Phase III
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
The purpose of the study is to examine the safety and efficacy of liposome bupivacaine for intercostal nerve block in subjects undergoing posterolateral thoracotomy.
Purpose
Intercostal Nerve Block With Liposome Bupivacaine in Subjects Undergoing Posterolateral Thoracotomy
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2012-12-01
Anticipated Date of Last Follow-up
2021-07-08
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2013-06-01
Actual Completion Date
2013-06-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female, ≥18 years of age. * Scheduled to undergo a thoracotomy of at least 3 inches (7.6 cm) of intercostal incisional length or requiring insertion of an inter-rib spreader/retractor for a primary thoracic non-infectious indication under general anesthesia. * American Society of Anesthesiologists (ASA) Physical Status 1 - 3. * Able to provide informed consent, adhere to the study visit schedule, and complete all study assessments. * Able to demonstrate sensory function by exhibiting sensitivity to cold in one dermatome area in which study drug will be administered. Exclusion Criteria: * Currently pregnant, nursing, or planning to become pregnant during the study or within 1 month after study drug administration. Female subjects must be surgically sterile.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
191
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
402-C-326
Identifier
NCT02713178
Link
https://clinicaltrials.gov/study/NCT02713178
Phase
Phase III
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
This is a Phase 3, multicenter, randomized, double-blind, placebo-controlled study in 232 adult subjects undergoing primary unilateral TKA under general or spinal anesthesia.
Purpose
Efficacy, Safety, and Pharmacokinetics of Femoral Nerve Block With EXPAREL in Total Knee Arthroplasty
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2016-06-03
Anticipated Date of Last Follow-up
2020-11-06
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2017-06-30
Actual Completion Date
2017-06-30
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Male or female, at least 18 years of age at screening. 2. Scheduled to undergo primary unilateral TKA under general or spinal anesthesia. 3. American Society of Anesthesiologists (ASA) physical status 1, 2, or 3. 4. Female subject must be surgically sterile; or at least 2 years postmenopausal; or have a monogamous partner who is surgically sterile; or practicing double-barrier contraception; or practicing abstinence.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
232
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
402-C-331
Identifier
NCT02713490
Link
https://clinicaltrials.gov/study/NCT02713490
Phase
Marketed
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
This is a Phase 4, multicenter, randomized, double-blind, controlled trial in approximately 140 adult subjects undergoing primary unilateral TKA under spinal anesthesia with bupivacaine HCl (10-15 mg).
Purpose
Local Infiltration Analgesia With and Without EXPAREL Following Total Knee Arthroplasty
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2016-04-18
Anticipated Date of Last Follow-up
2020-12-10
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2017-02-08
Actual Completion Date
2017-02-08
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Male
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Male or female, at least 18 years of age at screening. 2. Scheduled to undergo primary, unilateral, tricompartmental TKA under spinal anesthesia. 3. Primary indication for TKA is degenerative osteoarthritis of the knee. 4. American Society of Anesthesiologists (ASA) physical status 1, 2, or 3. 5. Female subjects must be surgically sterile; or at least 2 years postmenopausal; or have a monogamous partner who is surgically sterile; or practicing double-barrier contraception; or practicing abstinence (must agree to use double-barrier contraception in the event of sexual activity); or using an insertable, injectable, transdermal, or combination oral contraceptive approved by the FDA for greater than 2 months prior to screening and commit to the use of an acceptable form.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
140
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
AIMS
Identifier
NCT05730920
Link
https://clinicaltrials.gov/study/NCT05730920
Phase
Marketed
Status
Recruiting
Sponsor
Dr. Casey Stondell, MD
More details
The goal of this pilot study is to assess the feasibility of conducting a randomized controlled trial at a single institution comparing erector spinae plane blockade (ESPB) with liposomal bupivacaine (LB, Exparel) to intravenous (IV) methadone for managing pain in pediatric subjects undergoing adolescent and juvenile idiopathic scoliosis correction. Specifically, the goal is to enroll 15 subjects in each group and to complete data collection for all subjects. If this pilot study is successful, we plan to then design a larger scale study powered to compare specific outcomes between the two groups.
Purpose
IV Methadone vs EXPAREL Erector Spinae Plane Blockade in Pediatric Subjects Undergoing Idiopathic Scoliosis Correction
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-10-19
Anticipated Date of Last Follow-up
2024-04-15
Estimated Primary Completion Date
2024-06-01
Estimated Completion Date
2025-06-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Children
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female patients 11 to less than 18 years of age on the day of surgery. * Subjects whose parent(s) or guardian(s) has/have signed and dated the informed consent form (ICF) for the subject to participate in the study * Patients with a diagnosis of juvenile idiopathic scoliosis (JIS) or adolescent idiopathic scoliosis (AIS) who will have posterior spinal fusion surgery * American Society of Anesthesiologists (ASA) Class 1-2. * Able to adhere to the study visit schedule and complete all study assessments. Exclusion Criteria: * Body mass index ≥35 at the time of screening * Contraindication to regional anesthesia (e.g., abnormal coagulation, infection at site) * Current opioid use at the time of screening * Current diagnosis of chronic pain.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
30
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Single blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
14-903
Identifier
NCT04278846
Link
https://clinicaltrials.gov/study/NCT04278846
Phase
Marketed
Status
Completed
Sponsor
The Cleveland Clinic
More details
Patients undergoing breast reconstruction with tissue expanders will be treated with a local anesthetic during their procedure and monitor their post-operative pain level and amount of oral pain medication taken. This information will be used to determine if liposomal bupivacaine provides better and longer pain control vs. bupivacaine HCl.
Purpose
Efficacy of Liposomal Bupivacaine for Prolonged Postoperative Analgesia in Patient Undergoing Breast Reconstruction With Tissue Expander
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2014-08-22
Anticipated Date of Last Follow-up
2022-06-07
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2017-12-31
Actual Completion Date
2019-05-15
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Female
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * undergoing breast reconstruction with tissue expander Exclusion Criteria: * history of adverse reaction to local anesthesia * chronic liver disease * history of chronic preoperative consumption of narcotics or opioids * history of alcohol and/or illicit drug dependence * undergoing combined procedures * diagnosed with neuromuscular/neurosensory disorder * positive pregnancy test * previous breast conservation therapy (lumpectomy with radiation treatment * previous surgeries or trauma in the breast or chest region (denervation may bias pain perception) * axillary node dissection * psychosis.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
50
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
CHOICE
Identifier
NCT03853694
Link
https://clinicaltrials.gov/study/NCT03853694
Phase
Marketed
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
The purpose of this study is to compare total opioid consumption by subjects in different treatment groups. Another purpose of this study is to assess how well EXPAREL works, collect any safety data and assess your satisfaction using EXPAREL.
Purpose
Efficacy and Safety of EXPAREL Versus Standard of Care (SoC) in Subjects Undergoing Elective Cesarean Section
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-03-04
Anticipated Date of Last Follow-up
2022-07-15
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2020-01-09
Actual Completion Date
2020-01-16
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Female
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Females 18 years of age and older at screening. 2. Term pregnancies of 37 to 42 weeks gestation, scheduled to undergo elective C-section. 3. American Society of Anesthesiology (ASA) physical status 1, 2, or 3. 4. Able to provide informed consent, adhere to the study visit schedule, and complete all study assessments. Exclusion Criteria: 1. Subjects who, in the opinion of the study site principal investigator, have a high-risk pregnancy. 2. Subjects with a pregnancy-induced medical condition or complication. 3. Subjects with 3 or more prior C-sections. 4. Pre-pregnancy body mass index \>50 kg/m2. 5. Allergy, hypersensitivity, intolerance, or contraindication to any of the study medications. 6. Planned concurrent surgical procedure.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
167
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
IMPROVE-Open
Identifier
NCT01507246
Link
https://clinicaltrials.gov/study/NCT01507246
Phase
Marketed
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
This study is designed to compare the standard of care against EXPAREL(R) to determine if total opioid consumption is reduced when using EXPAREL, therefore possibly reducing total hospitalization costs.
Purpose
Adult Patients Undergoing Open Colectomy MA402S23B303
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2011-12-01
Anticipated Date of Last Follow-up
2013-05-06
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2012-07-01
Actual Completion Date
2012-08-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female, 18 years of age and older. * Patients scheduled to undergo open segmental colectomy with planned primary anastamosis, as defined by cecectomy, right hemicolectomy, resection of transverse colon, left hemicolectomy, or sigmoidectomy. * Ability to provide informed consent, adhere to study visit schedule, and complete all assessments. Exclusion Criteria: * Patients with a history of hypersensitivity or idiosyncratic reactions or intolerance to any local anesthetic, opioid, or propofol. * Patients who abuse alcohol or other drug substance. * Patients with severe hepatic impairment. * Patients currently pregnant or who may become pregnant during the course of the study. * Patients with any psychiatric, psychological, or other condition that the Investiga
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
42
Allocation
Randomized
Intervention model
Factorial assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
PRO00091425
Identifier
NCT03827291
Link
https://clinicaltrials.gov/study/NCT03827291
Phase
Marketed
Status
Completed
Sponsor
Duke University
More details
The purpose of this study is to determine if using a different type of injection of local anesthestic (pain medicine) in between the muscle layers of the abdominal wall (called a quadratus lumborum block) will improve pain control and be easier to manage after surgery than the current standard of care epidural (spinal injection) pain relief for patients undergoing laparoscopy colectomy.
Purpose
QL Block With Exparel in Colectomy
Interventions
Intervention 1
Intervention 2
Countries
Not provided
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-10-31
Anticipated Date of Last Follow-up
2022-12-19
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-12-02
Actual Completion Date
2022-12-09
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Patients scheduled for elective, laparoscopic colonic resection by one of three surgeons: Drs. Thacker, Mantyh or Migaly. These surgeons perform this procedure in the same manner * Age 18-85 years * American Society of Anesthesiologists (ASA) Physical Class I-III * BMI 18-35 kg/m\^2 Exclusion Criteria: * Inability to consent * Inability to speak English * Pregnancy * Emergency surgery * Contraindications to regional blockade: coagulopathy or bleeding diathesis, local infection, allergy to local anesthetics * Allergies/intolerances/contraindications to any of the multimodal agents (acetaminophen, gabapentin, ketorolac) * Daily opioid equivalent use of 30 mg of morphine or greater at time of consent * History of drug or alcohol abuse * Rheumatoid arthritis
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
42
Allocation
Not provided
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
402-C-405
Identifier
NCT02284386
Link
https://clinicaltrials.gov/study/NCT02284386
Phase
Marketed
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
This is a Phase 4, multicenter, open-label study designed to characterize the pharmacokinetic (PK) profile of total bupivacaine in approximately 15 adult subjects undergoing primary unilateral total knee arthroplasty (TKA) with bupivacaine hydrochloride (HCl) spinal nerve block (SNB) and EXPAREL local infiltration into the surgical site.
Purpose
Pharmacokinetics and Safety of Bupivacaine HCl Spinal Block and EXPAREL Local Infiltration in Total Knee Arthroplasty
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2014-12-01
Anticipated Date of Last Follow-up
2021-02-11
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2015-03-01
Actual Completion Date
2015-03-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Males or females ≥18 years of age. 2. American Society of Anesthesiologists (ASA) physical status 1, 2, or 3. 3. Scheduled to undergo spinal block in conjunction with unilateral TKA. 4. Female subjects must be surgically sterile, at least 2 years postmenopausal, or using a medically acceptable method of birth control. Females of childbearing potential must have a documented negative blood or urine pregnancy test result within 24 hours before surgery. 5. Able to provide informed consent, adhere to the study schedule, and complete all study assessments. Exclusion Criteria: 1. History of hypersensitivity or idiosyncratic reactions to amide-type local anesthetics or opioids. 2. Contraindication to bupivacaine.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
15
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
MA402S23B901
Identifier
NCT01582490
Link
https://clinicaltrials.gov/study/NCT01582490
Phase
Marketed
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
This is a Phase 4, prospective, open-label, non-randomized, sequential study with two treatment groups differing only in the technique used for EXPAREL administration (instillation or infiltration).
Purpose
Study of EXPAREL in Patients Undergoing Breast Augmentation
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2012-04-01
Anticipated Date of Last Follow-up
2014-05-31
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2012-11-01
Actual Completion Date
2012-11-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Female
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Female, 18-75 years of age inclusive. * American Society of Anesthesiologists (ASA) physical status 1-3. * Undergoing bilateral augmentation mammoplasty without any concurrent surgical procedure(s). * Physically and mentally able to participate in the study and complete all study assessments. * Able to give fully informed consent to participate in this study after demonstrating a good understanding of the risks and benefits of the study components. Exclusion Criteria: * History of hypersensitivity or idiosyncratic reactions to amide-type local anesthetics. * Any subject whose anatomy or surgical procedure might, in the opinion of the Investigator, preclude the potential successful local administration of EXPAREL.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
19
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
STUDY00000101
Identifier
NCT02128646
Link
https://clinicaltrials.gov/study/NCT02128646
Phase
Marketed
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
The purpose of this study is to learn how well Liposomal Bupivacaine (Exparel™) controls post-operative pain in patients undergoing both open and laparoscopic (minimally invasive) abdominal hernia repair surgery.
Purpose
Liposomal Bupivacaine (Exparel) for Postoperative Pain Control for Open and Laparoscopic Abdominal Hernia Repair
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Not provided
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2014-04-01
Anticipated Date of Last Follow-up
2017-01-17
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2017-01-01
Actual Completion Date
2017-01-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Patients scheduled to undergo an open or laparoscopic abdominal hernia repair. * Ability to provide informed consent, adhere to study visit schedule, and complete all study assessments. Exclusion Criteria: * Patients with a history of hypersensitivity or idiosyncratic reactions or intolerance to any local anesthetic, opioids or ketorolac. * Patients who abuse alcohol or other drug substance. * Patients who are on chronic opioid therapy (taken an opioid 5 of the last 7 days). * Patients with severe hepatic impairment. * Patients currently pregnant.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
40
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
IMPROVE-IR
Identifier
NCT01509638
Link
https://clinicaltrials.gov/study/NCT01509638
Phase
Marketed
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
This study is designed to compare the standard of care against EXPAREL (bupivacaine liposome injectable suspension) to determine if total opioid consumption is reduced when using EXPAREL, therefore possibly reducing total hospitalization costs.
Purpose
A Health Economic Trial in Adult Patients Undergoing Ileostomy Reversal MA402S23B501
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2012-03-01
Anticipated Date of Last Follow-up
2014-01-19
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2012-10-01
Actual Completion Date
2012-10-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female, 18 years of age or older. * Patients scheduled to undergo ileostomy reversal. * Ability to provide informed consent, adhere to study visit schedule, and complete all assessments. Exclusion Criteria: * Patients with a history of hypersensitivity or idiosyncratic reactions or intolerance to any local anesthetic, opioid, or propofol. * Patients who abuse alcohol or other drug substance. * Patients with severe hepatic impairment. * Patients currently pregnant or who may become pregnant during the course of the study or who are unwilling to use acceptable means of contraception for at least one month before and one month after dosing. * Patients with any psychiatric, psychological, or other condition.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
47
Allocation
Not provided
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
12-13-12E
Identifier
NCT02586077
Link
https://clinicaltrials.gov/study/NCT02586077
Phase
Marketed
Status
Completed
Sponsor
OrthoCarolina Research Institute, Inc.
More details
The purpose of this study is to review the use of surgeon applied liposomal release anesthetic (Exparel) for post operative analgesia. Hypothesis: Surgeon applied use of liposomal related anesthetic provides excellent post operative analgesia. Immediated and 72 hour narcotic use will be significantly impacted by the use of this modality.
Purpose
Foot and Ankle Clinic Application for Liposomal Related Anesthetic
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2014-02-01
Anticipated Date of Last Follow-up
2020-10-08
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2016-06-30
Actual Completion Date
2016-11-15
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Any patient scheduled for ankle arthrodesis, a tibiotalocalcaneal arthrodesis, hindfoot arthrodesis including subtalar, double, triple, or isolated talonavicular arthrodesis * Patients over the age of 18 * The subject is psychosocially, mentally, and physically able to understand and comply with the requirements of the study Exclusion Criteria: * \< 18 years of age * Patients with a history of infection * Patients diagnosed with neuropathy or any form of numbness or loss of feeling in the arms or legs due to damaged nerves * Patients having surgery on both feet at the same time * Patients having any other different type of foot and ankle surgery
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
36
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
PRO17020089
Identifier
NCT03149887
Link
https://clinicaltrials.gov/study/NCT03149887
Phase
Marketed
Status
Completed
Sponsor
Steven Orebaugh
More details
Liposomal bupivacaine, a long-acting form of bupivacaine, has been found to be effective for postoperative pain control after total knee, total hip and total shoulder arthroplasty. We are conducting a randomized, controlled trial to evaluate pain control after arthroscopic rotator cuff repair in ambulatory patients, comparing standard care in the control group, with standard care plus the addition of injection of liposomal bupivacaine in the experimental group.
Purpose
Liposomal Bupivacaine After Arthroscopic Rotator Cuff Repair
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2017-12-05
Anticipated Date of Last Follow-up
2020-01-05
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2019-02-26
Actual Completion Date
2019-03-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Adult patients up to age 65 years, undergoing elective, ambulatory, arthroscopic rotator cuff repair. Exclusion Criteria: * Pregnancy, coagulopathy, allergy to bupivacaine, renal failure, hepatic insufficiency, and/or inappropriate candidate for usual therapy (specifically, if unable to receive the usual preoperative interscalene nerve block: preexisting nerve injury on side of surgery, refusal of nerve block, infection at site of nerve block).
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
54
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
IRB-300002544
Identifier
NCT03738696
Link
https://clinicaltrials.gov/study/NCT03738696
Phase
Marketed
Status
Completed
Sponsor
University of Alabama at Birmingham
More details
To assess the efficacy of interscalene liposomal bupivacaine in controlling postoperative pain scores, oral morphine equivalents and sleep quality after arthroscopic rotator cuff repair surgery as compared to interscalene catheter.
Purpose
Liposomal Bupivacaine in Rotator Cuff Repair
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-12-15
Anticipated Date of Last Follow-up
2024-05-06
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-01-01
Actual Completion Date
2022-01-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Undergoing outpatient arthroscopic rotator cuff repair * Greater than or equal to 19 years of age at the time of surgery Exclusion Criteria: * Planned operative fixation of the biceps tendon or acromioclavicular joint * Opioid use 6 weeks before surgery * Gabapentin use 6 weeks before surgery * History of prior shoulder surgery on the operative limb * Severe pulmonary dysfunction * Diagnosis of chronic pain, fibromyalgia or other somatosensory disorder(s) * History of radicular pain or neuropathy in the operative limb * Patients who are currently incapacitated for medical decision making.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
64
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
702
Identifier
NCT01801124
Link
https://clinicaltrials.gov/study/NCT01801124
Phase
Marketed
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
Phase 4 study evaluating the effectiveness of EXPAREL when infiltrated into the the Transversus Abdominis Plane (TAP).
Purpose
EXPAREL Infiltrated Into the TAP for Postoperative Analgesia in Unilateral Abdominal Hernia Repair
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2012-04-01
Anticipated Date of Last Follow-up
2021-02-11
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2012-12-01
Actual Completion Date
2012-12-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Males and females, aged 18-75 years inclusive, and ASA physical status 1-3. * Undergoing open repair of a unilateral abdominal hernia below the level of the umbilicus. * Abdominal incision length of 3-12 cm. * Subjects must be physically and mentally able to participate in the study and complete all study assessments. * Subjects must be able to give fully informed consent to participate in this study after demonstrating a good understanding of the risks and benefits of the proposed components of the TAP infiltration. Exclusion Criteria: * History of hypersensitivity or idiosyncratic reactions to amide-type local anesthetics * Any subject whose anatomy, or surgical procedure, in the opinion of the Investigator, might preclude the potential successful performance of
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
13
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
18-133
Identifier
NCT03541941
Link
https://clinicaltrials.gov/study/NCT03541941
Phase
Marketed
Status
Completed
Sponsor
The Cleveland Clinic
More details
Patients usually experience some level of pain after their hernia repair. To control pain after the operation, surgeons have many options. One of them is to make some injections of pain blocker medications into the nerves that are responsible for the sensations the abdominal wall.This procedure is called TAP block (transversus abdominis place block). These medications are called local anesthetics, and there is a variety of medications that can be used. One of such medications is called Exparel® (Liposomal Bupivacaine). Exparel® has the potential benefit of lasting more hours than regular anesthetics. Although this drug is being used with increasing frequency, the investigators do not have good quality studies investigating the benefits of using this medication during a hernia repair, espec
Purpose
Exparel vs. Bupivacaine Hydrochloride vs. Placebo for Hernia Repair
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Not provided
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2018-07-03
Anticipated Date of Last Follow-up
2021-09-15
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2019-12-19
Actual Completion Date
2019-12-19
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Patients with Primary Ventral or Incisional Hernias * Scheduled to undergo hernia repair through an open approach * Hernia repair performed in an elective setting * Hernia repair performed through a midline incision * Hernia repair performed in a clean wound * Hernia repair performed with mesh placed in the retromuscular position Exclusion Criteria: * patients with less than 18 years old of age * patients scheduled to undergo a minimally invasive hernia repair * patients where hernia repair is planned to be performed with mesh placement in a different position than retromuscular * patient undergoing hernia repair with a clean-contaminated, contaminated or infected wound * patients undergoing hernia repair in an non-elective fashion * patients with allergy, hypersen
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
164
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
C-Section
Identifier
NCT03176459
Link
https://clinicaltrials.gov/study/NCT03176459
Phase
Marketed
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
Primary objective: The primary objective of this study is to compare total opioid consumption through 72 hours following EXPAREL+bupivacaine HCl infiltration into the transversus abdominis plane (TAP) after spinal anesthesia to active bupivacaine HCl TAP infiltration after spinal anesthesia in subjects undergoing an elective cesarean section (C-section). Secondary objective: The secondary objectives are to assess efficacy and safety parameters and patient satisfaction.
Purpose
Evaluate the Safety and Efficacy of EXPAREL When Administered Via Infiltration Into the TAP vs. Bupivacaine Alone in Subjects Undergoing Elective C-Sections
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2017-06-01
Anticipated Date of Last Follow-up
2020-12-10
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2018-11-20
Actual Completion Date
2018-12-04
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Female
Accepts pregnant individuals
Yes
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: 1. Females 18 years of age and older at screening. 2. Term pregnancies of 37 to 42 weeks gestation, scheduled to undergo elective C-section. 3. American Society of Anesthesiology (ASA) physical status 1, 2, or 3. 4. Able to provide informed consent, adhere to the study visit schedule, and complete all study assessments. Exclusion Criteria: 1. Subjects who, in the opinion of the study site principal investigator, have a high-risk pregnancy (eg, multiple gestations, pregnancy resulting from in vitro fertilization, gestational diabetes, end-term prolonged bed rest required for medical reasons). 2. Subjects with a pregnancy-induced medical condition or complication (eg, hypertension, pre-eclampsia, chorioamnionitis). 3. Subjects with 3 or more prior C-sections.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
186
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
402-C-406
Identifier
NCT02255500
Link
https://clinicaltrials.gov/study/NCT02255500
Phase
Marketed
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
The purpose of this study is to evaluate the safety and pharmacokinetics of EXPAREL in subjects who undergo femoral nerve block with bupivacaine HCl for unilateral total knee arthroplasty (TKA).
Purpose
EXPAREL Infiltration in Subjects Undergoing Femoral Nerve Block With Bupivacaine HCl for Total Knee Arthroplasty
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2014-09-01
Anticipated Date of Last Follow-up
2021-02-11
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2015-03-01
Actual Completion Date
2015-03-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Males or females ≥18 years of age. 2. American Society of Anesthesiologists (ASA) physical status 1, 2, or 3. 3. Scheduled to undergo femoral nerve block in conjunction with unilateral TKA. 4. Female subjects must be surgically sterile, at least 2 years postmenopausal, or using a medically acceptable method of birth control. Females of childbearing potential must have a documented negative blood or urine pregnancy test result within 24 hours before surgery. 5. Able to provide informed consent, adhere to the study schedule, and complete all study assessments. Exclusion Criteria: 1. History of hypersensitivity or idiosyncratic reactions to amide-type local anesthetics or opioids. 2. Contraindication to bupivacaine. 3. Received bupivacaine
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
23
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
IMPROVE-IR
Identifier
NCT01509807
Link
https://clinicaltrials.gov/study/NCT01509807
Phase
Marketed
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
This study is designed to compare the standard of care against EXPAREL (bupivacaine liposome injectable suspension) to determine if total opioid consumption is reduced when using EXPAREL, therefore possibly reducing total hospitalization costs.
Purpose
A Health Economic Trial in Adult Patients Undergoing Ileostomy Reversal MA402S23B504
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2012-01-01
Anticipated Date of Last Follow-up
2014-03-09
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2012-10-01
Actual Completion Date
2012-10-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female, 18 years of age and older * Patients scheduled to undergo ileostomy reversal * Ability to provide informed consent, adhere to study visit schedule, and complete all assessments. Exclusion Criteria: * Patients with a history of hypersensitivity or idiosyncratic reactions or intolerance to any local anesthetic, opioid, or propofol. * Patients who abuse alcohol or other drug substance. * Patients with severe hepatic impairment. * Patients currently pregnant or who may become pregnant during the course of the study or who are unwilling to use acceptable means of contraception for at lest one month before and one month after dosing. * Patients with any psychiatric, psychological, or other condition.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
33
Allocation
Not provided
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
TAP
Identifier
NCT01582477
Link
https://clinicaltrials.gov/study/NCT01582477
Phase
Marketed
Status
Completed
Sponsor
Pacira Pharmaceuticals, Inc
More details
The purpose of the study is to assess the safety and efficacy of EXPAREL when administered via infiltration into the transversus abdominis plane (TAP) to prolonged postsurgical analgesia in men undergoing robot-assisted laparoscopic prostatectomy.
Purpose
TAP-patients With Robotic Assisted Lap Prostatectomy
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2012-03-01
Anticipated Date of Last Follow-up
2021-06-11
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2012-05-01
Actual Completion Date
2012-07-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Male
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * male subjects, aged 18-75. * American Society of Anesthesiology (ASA) physical status 1-3. * Undergoing robot-assisted laparoscopic prostatectomy performed by a single surgeon (Ingolf Tuerk, MD). * Subjects must be physically and mentally able to participate in the study and complete all study assessments. * Subjects must be able to give fully informed consent to participate in this study after demonstrating a good understanding of the risks and benefits of the TAP infiltration. Exclusion Criteria: * Demonstrated hypersensitivity or idiosyncratic reactions to amide-type local anesthetics. * Inability to tolerate oxycodone with acetaminophen (e.g. Percocet).
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
24
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PEP
Key resources
Excipients
Proprietary excipients used
Not provided
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
Not provided
Residual solvents used
Not provided
Additional features
Other features of the technology
- Biodegradable
- Drug-eluting
- Reservoir-type
- At least 1 year shelf life
Release properties
The release pattern of API from the pMVL matrix is triphasic. The pMVL technology holds promise in extending the release of the drug to the target site within an effective dosage range, spanning from 26 hours to 90 days, via the liposomal matrix with an initial burst from the surface vesicle within 20 hours of administration.
Injectability
pMVL preparations can be injected using a 25 gauge or larger-bore needle to ensure the preservation of structural integrity for liposomal bupivacaine particles. Moreover, pMVL products presents diverse injectable formats, including but not limited to Intravenous, Epidural, Intraperitoneal, Subcutaneous, Intramuscular, Ocular, and Intrathecal administrations.
Safety
The results of Phase III clinical trials of FDA-approved Exparel (300 mg and 600 mg) showed that there were no severe adverse drug reactions. However, 50-60% of participants experienced common adverse reactions similar to those seen with the generic drug.
Stability
Proprietary multivesicular liposome (pMVL) products offer better stability, with the potential to remain viable for up to two years if left unopened.
Storage conditions and cold-chain related features
pMVL formulations are typically refrigerated at temperatures ranging from 2°C to 8°C. They can be stored at a controlled room temperature of 20°C to 25°C for a maximum of 30 days in sealed, intact (unopened) vials. Avoid freezing or exposing the vials to temperatures exceeding 40°C for prolonged periods. Before administration, allow the vials to reach room temperature (20°C to 25°C) for up to 4 hours.
Potential application(s)
Therapeutic area(s)
Use case(s)
Use of technology
Ease of administration
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
Frequency of administration
Single Dose administration lasting for 72 hours, Weekly
User acceptance
Not provided
Targeted user groups
Age Cohort- Adults
- Older Adults
- All
Pregnant individuals
Yes
Lactating individuals
Unspecified
Healthy individuals
Unspecified
Comment
Not provided
Potential associated API(s)
Bupivacaine Hydrochloride
Class(es)
Anaesthetics
Development stage
Marketed
Clinical trial number(s)
NCT05157841
Foreseen/approved indication(s)
Postsurgical pain management
Foreseen user group
Patients who are older than 6 years
Foreseen duration between application(s)
Single dose administration (lasts up to 72 hours)
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Exparel has received approval from the United States Food and Drug Administration and marketing authorization from the European Medicines Agency. It is indicated for post-surgical analgesia.
Cytarabine
Class(es)
Antineoplastic Agent
Development stage
Marketed
Clinical trial number(s)
NCT00029523
Foreseen/approved indication(s)
Lymphomatous meningitis
Foreseen user group
Patients who are older than 3 years
Foreseen duration between application(s)
Every 14 days for 2/3 doses
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
DepoCyt has been approval in the United States Food and Drug Administration and received marketing authorization from the European Medicines Agency.
Patent info
Description
Method for Formulating Multivesicular Liposomes
Brief description
The present invention generally relates to the field of pharmaceutical sciences. More specifically, the present invention relates to an evaporation apparatus and a process for the preparation of multivesicular liposomes (MVL) using such an apparatus and the process of formulating the Liposomal injection.
Representative patent
WO2011127456
Category
Formulation
Patent holder
Pacira Pharmaceuticals
Exclusivity
Not provided
Expiration date
April 8, 2031
Status
Not provided
Description
Sustained Release Anesthetic Compositions
Brief description
The invention provides a method for obtaining local anesthetics encapsulated in liposomes, such as multivesicular liposomes, with high encapsulation efficiency and slow release in vivo. When the encapsulated anesthetic is administered as a single intravenous dose, the duration of anesthesia and the half-life of the drug at the local site of action are increased compared to the injection of unencapsulated anesthetic. The maximum tolerated dose of encapsulated anesthetic is also markedly increased in the liposomal formulation compared to the injection of unencapsulated anesthetic. These results show that the liposomal formulation of local anesthetic is useful for sustained local infiltration and nerve block anesthesia.
Representative patent
USOO8182835B2
Category
Not provided
Patent holder
Pacira Pharmaceuticals, Inc
Exclusivity
Not provided
Expiration date
April 1, 2025
Status
Not provided
Supporting material
Publications
<p><span style="color: rgb(33, 33, 33);">Angst, M. S., & Drover, D. R. (2006). Pharmacology of drugs formulated with DepoFoam: a sustained release drug delivery system for parenteral administration using multivesicular liposome technology. </span><em style="color: rgb(33, 33, 33);">Clinical pharmacokinetics</em><span style="color: rgb(33, 33, 33);">, </span><em style="color: rgb(33, 33, 33);">45</em><span style="color: rgb(33, 33, 33);">(12), 1153–1176. https://doi.org/10.2165/00003088-200645120-00002</span></p>
Multivesicular liposomes are structurally distinct from lamellar liposomes and consist of an aggregation of hundreds of water-filled polyhedral compartments separated by bi-layered lipid septa. The unique architecture of multivesicular liposomes allows encapsulating drug with greater efficiency, provides robust structural stability and ensures reliable, steady and prolonged drug release. The favourable characteristics of multivesicular liposomes have resulted in many drug formulations exploiting this technology, which is proprietary and referred to as DepoFoam™.
Additional documents
No documents were uploaded
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Illustrations

Release of the API molecules from the pMVL matrix
PMVL (no date) Pacira. Available at: https://www.pacira.com/products/pmvl (Accessed: 14 May 2024).