
|
Developed by 

|
Supported by 

|
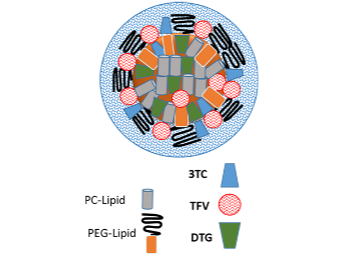
Drug Combination Nanoparticles (DcNP)
Verified by the innovator, on Jul 2021Developer(s)

|
Targeted & Long-acting drug Combination (TLC) Program, University of Washington Originator
https://depts.washington.edu/tlcart/
United States The purpose of the Targeted Long-acting Combination AntiRetroviral Therapy (TLC-ART) program is to develop one or more safe, stable, scalable and tolerable long acting antiretroviral combinations for treatment of HIV infection. |
Sponsor(s)

|
Unitaid https://unitaid.org |
Partnerships

|
Clinton Health Access Initiative https://www.clintonhealthaccess.org/ |

|
Medicines Patent Pool https://medicinespatentpool.org/ |
Technology information
Type of technology
Based on other organic particles, Aqueous drug particle suspension
Administration route
Subcutaneous
Development state and regulatory approval
Lamivudine (3TC), Dolutegravir (DTG), Tenofovir (TFV)
Phase I
Not provided
Description
A targeted and long-acting Drug combination Nano-Particle (DcNP) platform that enables transformations of existing and new drug entities from short-acting daily doses to a long-acting drug combination for a maximum therapeutic effect through sustained viral suppression.
Technology highlight
TLC's enabling DcNP technology has been validated to work with a number of current HIV drug combinations using clinics in both pediatric and adult populations. A number of these formulations have been evaluated in non-human primate models to provide both plasma and cell drug levels that persist for weeks after a single subcutaneous injection. This technology has been shown to bring together water soluble (such as tenofovir and lamivudine) and insoluble (such as lopinavir, ritonavir, atezenovir, and dolutegravir) drugs into an all-in-one long-acting injectable product. At least 4 formulations have been tested in NHP to have long-acting plasmakinetics for all drugs and are targeted to enhanced drug levels above plasma drug combinations in HIV host cells-PBMCs.
Technology main components
Drug API and common lipid excipients used in pharmaceutical formulations.
Information on the raw materials sourcing, availability and anticipated price
The existing HIV drug APIs can be sourced through WHO pre-qualified raw material suppliers.
Delivery device(s)
No delivery device
APIs compatibility profile
API desired features
Water-soluble molecules
Water-insoluble molecules
Small molecules
Tenofovir, Lamivudine, Dolutegravir, Lopinavir, Ritonavir, Atazanavir, Efavirenz
Additional solubility data
Not provided
Additional stability data
Not provided
API loading: Maximum drug quantity to be loaded
10-30 wt%
API co-administration
4 different APIs : 2 to 4 different drugs in a single injection are being investigated
LogP
Not provided
Scale-up and manufacturing prospects
Scale-up prospects
Good
Tentative equipment list for manufacturing
Spray-dryer; homogenization; size-reduction
Manufacturing
Injectable cGMP facilties
Specific analytical instrument required for characterization of formulation
Not provided
Clinical trials
First in Human Clinical Trial of a Next Generation, Long-acting Injectable, Combination Antiretroviral Therapy Platform TLC-ART 101 (ACTU 2001)
Identifier
NCT05850728
Link
https://clinicaltrials.gov/study/NCT05850728?intr=TLC-ART&rank=1
Phase
Phase I
Status
Recruiting
Sponsor
NIH
More details
This study is a prospective, open-label, single-site, first-in-human study of a long-acting, injectable combination antiretroviral therapy platform, with a pharmacologically-guided adaptive design for dose escalation, de-escalation, and study duration. The study is designed to learn whether the formulation can be used as a platform for other drugs for treatment of HIV. The formulation is a drug combination nanoparticle (DCNP). The study will be conducted by UW Positive Research. The sample size for this study is 12-16. The study population consists of healthy adults without HIV. The study duration is 57 days per participant at the start of the study.
Purpose
Safety, tolerability and PK of single subcutaneous injection of TLC-ART 101 in healthy adults
Interventions
Intervention 1
Countries
Sites / Institutions
Trials dates
Anticipated Start Date
2023-04-01
Actual Start Date
2023-04-01
Anticipated Date of Last Follow-up
Not provided
Estimated Primary Completion Date
2025-10-25
Estimated Completion Date
2025-12-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
No
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
18 Years to 65 Years (Adult, Older Adult ) of any sex
Health status
Study type
Interventional (clinical trial)
Enrollment
16
Allocation
Non-randomized
Intervention model
Single group assignment
Intervention model description
This study is a prospective, open-label, single-site, first-in-human study of a long-acting, injectable combination antiretroviral therapy platform, with a pharmacologically-guided adaptive design for dose escalation, de-escalation, and study duration. The study is designed to learn whether the formulation can be used as a platform for other drugs for treatment of HIV. The formulation is a drug combination nanoparticle (DCNP). The study will be conducted by UW Positive Research. The sample size for this study is 12-16. The study population consists of healthy adults without HIV. The study duration is 57 days per participant at the start of the study.
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
Excipients
Proprietary excipients used
No proprietary excipient used
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
No novel excipient or existing excipient used
Residual solvents used
No residual solvent used
Additional features
Other features of the technology
- Biodegradable
- Non-removable
- Room temperature storage
- At least 1 year shelf life
Release properties
Intracellular uptake and accessible by cellular enzymes to transform into active metabolites such as TFV-DP and 3TC-TP.
Injectability
Not provided
Safety
Not provided
Stability
Not provided
Storage conditions and cold-chain related features
No cold-chain needed
Potential application(s)
Therapeutic area(s)
Use case(s)
Use of technology
Ease of administration
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
- Self-administered
Frequency of administration
Monthly
User acceptance
Not provided
Targeted user groups
Age Cohort- Adults
- Male
- Female
- Cisgender female
- Cisgender male
- Transgender female
- Transgender male
- Intersex
- Gender non-binary
- All
Pregnant individuals
Unspecified
Lactating individuals
Unspecified
Healthy individuals
No
Comment
Not provided
Potential associated API(s)
Lamivudine (3TC), Dolutegravir (DTG), Tenofovir (TFV)
Class(es)
Antiviral
Development stage
Phase I
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Treatment for people living with HIV
Foreseen user group
People living with HIV
Foreseen duration between application(s)
1 month
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Lopinavir and ritonavir (LPV/r), Tenofovir (TFV)
Class(es)
antiviral
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Treatment for people living with HIV to provide sustained viral suppression
Foreseen user group
People living with HIV
Foreseen duration between application(s)
1 month
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Tenofovir-Lamivudine-Dolutegravir (TLD) - long-acting injectable (LAI) (TLD LAI)
Class(es)
Not provided
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
HIV treatment
Foreseen user group
Adults living with HIV
Foreseen duration between application(s)
1 month
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Patent info
Technology patent families
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Antiviral compositions that transform short-acting therapeutic agents into long-acting injectable forms that lasts for many weeks per administration
Expiry date: 2041-01-07 The present disclosure describes simple, stable, and scalable antiviral therapeutic agent compositions that transform short-acting antiviral (e.g., anti-HIV) therapeutic agents that would otherwise require daily short-acting oral administration into long-acting injectable forms that lasts for many weeks per administration. A mixture of water-soluble and water-insoluble antiviral therapeutic agents can be present in the long-acting and drug-combination composition. |
WO2021142150 | University of Washington | Yes | Company |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | ||
| Filed | China, Brazil | United States of America |
| Not in force | World Intellectual Property Organization (WIPO) | World Intellectual Property Organization (WIPO) |
MPP Licence(s)
MPP/Uni Washington licence on Drug Combination Nanoparticles (DcNP) - HIV prevention/treatment
https://medicinespatentpool.org/licence-post/long-acting-injectable-drug-combination-for-hiv-treatment-preventionPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Combination pharmaceutical compositions including a combination of hydrophilic and hydrophobic therapeutic agents
Expiry date: 2040-01-10 The current invention is about a pharmaceutical formulation with a hydrophilic and hydrophobic drug along with a compatibilizer (lipid). The method of preparation is by dissolving all the components in suitable mixture of solvents, and then evaporating the solvent to get the powder form. |
WO2020146788 | University of Washington | Yes | Company |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | South Africa | |
| Filed | China, Brazil, India, Nigeria | |
| Not in force | World Intellectual Property Organization (WIPO) | World Intellectual Property Organization (WIPO), United States of America |
MPP Licence(s)
MPP/Uni Washington licence on Drug Combination Nanoparticles (DcNP) - HIV prevention/treatment
https://medicinespatentpool.org/licence-post/long-acting-injectable-drug-combination-for-hiv-treatment-preventionPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Drug Combination Nanoparticles for multiple drugs extended release
Expiry date: 2036-06-15 Multi-drug lipid nanoparticles that stably incorporate multiple small molecule drugs with divergent hydrophobic and water solubility characteristics and related methods of making and using the same. The disclosed compositions and methods provide for enhanced stability of lipid nanoparticle drug formulations that can reliably provide drugs addressing different mechanistic targets with prolonged presence in the body for more efficacious treatment and avoidance of single drug resistance. |
WO2016205384 | The University Of Washington | Yes | Company |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | United States of America | |
| Filed | World Intellectual Property Organization (WIPO), China | World Intellectual Property Organization (WIPO) |
| Not in force | China |
MPP Licence(s)
MPP/Uni Washington licence on Drug Combination Nanoparticles (DcNP) - HIV prevention/treatment
https://medicinespatentpool.org/licence-post/long-acting-injectable-drug-combination-for-hiv-treatment-preventionTenofovir/Dolutegravir/Lamivudine (LAI candidate)
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Emtricitabine and lamivudine compounds
Expiry date: 2010-02-08 Novel substituted 1,3-oxathiolane cyclic compounds having pharmacological activity, to processes for and intermediates of use in their preparation, to pharmaceutical compositions containing them, and to the use of these compounds in the antiviral treatment of mammals. |
CA2009637 | Compound | Biochem Pharma Inc, Iaf Biochem International, Inc | No | Health Canada |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | United States of America, Australia, Germany, Spain, Finland, Ireland, Portugal | |
| Filed | United States of America, Australia, Cyprus, Germany, Denmark, Spain, Greece, Hungary, Ireland, Japan, Luxembourg, Latvia, Netherlands, New Zealand, Poland, Singapore, Slovenia, Slovakia | |
| Not in force | Bosnia and Herzegovina, Yugoslavia/Serbia and Montenegro, China, Honduras, South Africa, Botswana, Ghana, Gambia (the), Kenya, Lesotho, Malawi, Sudan, Eswatini, Uganda, Zambia, Zimbabwe, Burkina Faso, Benin, Central African Republic, Congo, Côte d'Ivoire, Cameroon, Gabon, Guinea, Mali, Mauritania, Niger, Senegal, Chad, Togo, Malaysia, Philippines, Armenia, Kyrgyzstan, Tajikistan, Sri Lanka, Dominican Republic, Georgia, Uzbekistan, Mexico, Moldova, Republic of, Ukraine | Canada, United States of America, Austria, Germany, Denmark, Spain, Greece, Hong Kong, Croatia, Israel, Japan, Korea, Republic of, Luxembourg, Netherlands, Norway, Belgium, Switzerland, France, United Kingdom, Italy, Liechtenstein, Sweden, Uruguay, Saudi Arabia |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Emtricitabine and lamivudine - process for preparing
Expiry date: 2012-12-21 The present invention relates to processes for preparing substituted 1,3-oxathiolanes with antiviral activity and intermediates of use in their preparation. |
WO9414802 | Process | Biochem Pharma Inc | No | EPO (extended family) |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | ||
| Filed | Denmark, Spain, Portugal | |
| Not in force | World Intellectual Property Organization (WIPO) | Canada, Austria, World Intellectual Property Organization (WIPO), Australia, Germany, Denmark, Spain, Hungary, Japan, Portugal, Belgium, Switzerland, France, United Kingdom, Greece, Ireland, Italy, Liechtenstein, Luxembourg, Monaco, Netherlands, Sweden |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Lamivudine compound
Expiry date: 2011-05-02 (-)-4-Amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one, its pharmaceutically acceptable derivatives, pharmaceutical formulations thereof, methods for its preparation and its use as an antiviral agent are described. |
WO9117159 | Compound | Iaf Biochem International Inc | No | Health Canada |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | United States of America | |
| Filed | United Kingdom, Australia, Finland, Hungary, Japan, Korea, Republic of, New Zealand, Poland, Singapore, Slovenia | |
| Not in force | World Intellectual Property Organization (WIPO), Bosnia and Herzegovina, Yugoslavia/Serbia and Montenegro, China, Morocco, Moldova, Republic of, Tunisia, South Africa, Botswana, Ghana, Gambia (the), Kenya, Lesotho, Malawi, Sudan, Eswatini, Uganda, Zambia, Zimbabwe, Burkina Faso, Benin, Central African Republic, Congo, Côte d'Ivoire, Cameroon, Gabon, Guinea, Mali, Mauritania, Niger, Senegal, Chad, Togo, Egypt, Ukraine, Malaysia, Georgia | Canada, United Kingdom, World Intellectual Property Organization (WIPO), Bulgaria, Hong Kong, Croatia, Ireland, Israel, Japan, Korea, Republic of, Norway, Portugal, Romania, Slovakia, Taiwan, Province of China, United States of America, Austria, Belgium, Switzerland, Germany, Denmark, Spain, France, Greece, Italy, Liechtenstein, Luxembourg, Netherlands, Sweden |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Lamivudine crystal forms
Expiry date: 2012-06-02 (-)$i(cis)-4-Amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(IH)-pyrimidine-2-one in crystalline form, in particular as needle-shaped or bipyramidyl crystals, pharmaceutical formulations thereof, methods for their preparation and their use in medicine. |
WO9221676 | Polymorphs | Glaxo Group Limited | No | Health Canada, US FDA |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | United States of America | |
| Filed | United Kingdom, Austria, Australia, Denmark, Ireland, Iceland, Portugal, Singapore | |
| Not in force | Mexico, South Africa, Botswana, Ghana, Gambia (the), Kenya, Lesotho, Malawi, Sudan, Eswatini, Uganda, Zambia, Zimbabwe, Burkina Faso, Benin, Central African Republic, Congo, Côte d'Ivoire, Cameroon, Gabon, Guinea, Mali, Mauritania, Niger, Senegal, Chad, Togo, Philippines, Ukraine, Pakistan, Georgia, World Intellectual Property Organization (WIPO) | Canada, United Kingdom, Austria, Bulgaria, Germany, Denmark, Hong Kong, Ireland, Israel, Japan, Korea, Republic of, Norway, New Zealand, Portugal, Russian Federation, Slovakia, Taiwan, Province of China, Belgium, Switzerland, Spain, France, Greece, Italy, Liechtenstein, Luxembourg, Monaco, Netherlands, Sweden, World Intellectual Property Organization (WIPO) |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Tenofovir disoproxil compounds
Expiry date: 2017-07-25 The present invention relates to intermediates for phosphonomethoxy nucleotide analogs, in particular intermediates suitable for use in the efficient oral delivery of such analogs. |
WO9804569 | Compound | Gilead Sciences, Inc | Yes | Health Canada, US FDA, MPP Licence |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Luxembourg, United Kingdom | |
| Filed | ||
| Not in force | China, India, World Intellectual Property Organization (WIPO) | Canada, Austria, Australia, Germany, Denmark, Spain, Hong Kong, Japan, Korea, Republic of, Luxembourg, Netherlands, New Zealand, Portugal, Taiwan, Province of China, United States of America, Belgium, Switzerland, Finland, France, Greece, Ireland, Italy, Liechtenstein, Monaco, Sweden, Chile, World Intellectual Property Organization (WIPO), Singapore |
MPP Licence(s)
MPP licence on tenofovir disoproxil fumarate (TDF)
https://medicinespatentpool.org/licence-post/tenofovir-disoproxil-fumarate-tdf/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Tenofovir disoproxil fumarate (TDF)
Expiry date: 2018-07-23 The invention provides a composition comprising bis(POC)PMPA and fumaric acid (1:1). The composition is useful as an intermediate for the preparation of antiviral compounds, or is useful for administration to patients for antiviral therapy or prophylaxis. The composition is particularly useful when administered orally. The invention also provides methods to make PMPA and intermediates in PMPA synthesis. Embodiments include lithium t-butoxide, 9-(2-hydroxypropyl) adenine and diethyl p-toluenesulfonylmethoxy-phosphonate in an organic solvent such as DMF. The reaction results in diethyl PMPA preparations containing an improved by-product profile compared to diethyl PMPA made by prior methods |
WO9905150 | Compound, Salt | Gilead Sciences, Inc | Yes | Health Canada, US FDA, MPP Licence |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | ||
| Filed | Portugal | |
| Not in force | Brazil, China, India, Mexico, Indonesia, World Intellectual Property Organization (WIPO), Albania | Canada, United States of America, Austria, Australia, Germany, Denmark, Spain, Hong Kong, Japan, Korea, Republic of, New Zealand, Portugal, Singapore, Slovenia, Taiwan, Province of China, Belgium, Switzerland, Cyprus, Finland, France, United Kingdom, Greece, Ireland, Italy, Liechtenstein, Luxembourg, Monaco, Netherlands, Sweden, World Intellectual Property Organization (WIPO), Lithuania, Latvia, Romania |
MPP Licence(s)
MPP licence on tenofovir disoproxil fumarate (TDF)
https://medicinespatentpool.org/licence-post/tenofovir-disoproxil-fumarate-tdf/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Tenofovir alafenamide fumarate (TAF)
Expiry date: 2021-07-20 A novel method is provided for screening prodrugs of methoxyphosphonate nucleotide analogues to identify prodrugs selectively targeting desired tissues with antiviral or antitumor activity. This method has led to the identification of novel mixed ester-amidates of PMPA for retroviral or hepadnaviral therapy, including compounds of structure (5a) having substituent groups as defined herein. Compositions of these novel compounds in pharmaceutically acceptable excipients and their use in therapy and prophylaxis are provided. Also provided is an improved method for the use of magnesium alkoxide for the preparation of starting materials and compounds for use herein. |
WO0208241 | Compound | Gilead Sciences, Inc | No | Health Canada, US FDA |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Ukraine, Albania, Ethiopia, Fiji, Grenada, Kiribati, Solomon Islands, Saint Lucia | Australia, Bulgaria, Croatia, Israel, Iceland, Japan, Korea, Republic of, Norway, Poland, Russian Federation, Austria, Belgium, Switzerland, Cyprus, Germany, Denmark, Spain, Finland, France, United Kingdom, Greece, Ireland, Italy, Liechtenstein, Luxembourg, Netherlands, Sweden, Lithuania, Romania, Latvia, Slovenia, Estonia, Brunei Darussalam, Czechia, Anguilla, Bermuda, Falkland Islands (Malvinas), Montserrat, Turks and Caicos Islands, Virgin Islands (British), Cayman Islands, Gibraltar, Guernsey, Hungary |
| Filed | Jamaica | Norway |
| Not in force | China, Mexico, Türkiye, South Africa, Ghana, Gambia (the), Kenya, Lesotho, Malawi, Mozambique, Sudan, Sierra Leone, Eswatini, Tanzania, United Republic of, Uganda, Zimbabwe, Armenia, Azerbaijan, Belarus, Kyrgyzstan, Kazakhstan, Moldova, Republic of, Tajikistan, Turkmenistan, Burkina Faso, Benin, Central African Republic, Congo, Côte d'Ivoire, Cameroon, Gabon, Guinea, Equatorial Guinea, Guinea-Bissau, Mali, Mauritania, Niger, Senegal, Chad, Togo, India, Indonesia, Viet Nam, World Intellectual Property Organization (WIPO), North Macedonia, Albania, Congo, democratic Republic of the, Haiti, Nepal, Tuvalu, Brazil | Canada, Australia, Hong Kong, Croatia, Japan, Korea, Republic of, Norway, New Zealand, United States of America, Austria, Belgium, Switzerland, Cyprus, Germany, Denmark, Spain, Finland, France, United Kingdom, Greece, Ireland, Italy, Liechtenstein, Luxembourg, Monaco, Netherlands, Portugal, Sweden, World Intellectual Property Organization (WIPO), Lithuania, Romania, Latvia, Slovenia, Czechia, Guyana, Seychelles, Saint Helena, Ascension and Tristan da Cunha, Singapore, Jersey |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Dolutegravir and Cabotegravir compounds
Expiry date: 2026-04-28 The present invention is to provide a novel compound (I), having the anti-virus activity, particularly the HIV integrase inhibitory activity, and a drug containing the same, particularly an anti-HIV drug, as well as a process and an intermediate thereof. Compound (I) wherein Z<1> is NR<4>; R<1> is hydrogen or lower alkyl; X is a single bond, a hetero atom group selected from O, S, SO, SO2 and NH, or lower alkylene or lower alkenylene in which the hetero atom group may intervene; R<2> is optionally substituted aryl; R<3> is hydrogen, a halogen, hydroxy, optionally substituted lower alkyl etc; and R<4> and Z<2> part taken together forms a ring, to form a polycyclic compound, including e.g., a tricyclic or tetracyclic compound. |
WO2006116764 | Compound | Glaxosmithkline Llc | Yes | US FDA, Health Canada |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Brazil, China, Morocco, Mexico, Philippines, Ukraine, Viet Nam, South Africa, Türkiye, Armenia, Azerbaijan, Belarus, Kyrgyzstan, Kazakhstan, Moldova, Republic of, Tajikistan, Turkmenistan, Nigeria, Colombia, Indonesia, Malaysia, Algeria | United States of America, Australia, Canada, Cyprus, Hong Kong, Israel, Japan, Korea, Republic of, Luxembourg, Norway, New Zealand, Taiwan, Province of China, Austria, Belgium, Bulgaria, Switzerland, Czechia, Germany, Denmark, Estonia, Spain, Finland, France, United Kingdom, Greece, Hungary, Ireland, Iceland, Italy, Liechtenstein, Lithuania, Latvia, Monaco, Netherlands, Poland, Portugal, Romania, Sweden, Slovenia, Slovakia, Russian Federation, Trinidad and Tobago, Singapore |
| Filed | Egypt | United States of America, Cyprus, Luxembourg, Norway, Belgium, Finland, France, Hungary, Lithuania, Netherlands, Slovenia |
| Not in force | Türkiye, India, World Intellectual Property Organization (WIPO) | United States of America, Cyprus, Hong Kong, Israel, Japan, Luxembourg, Austria, Belgium, Bulgaria, Switzerland, Czechia, Germany, Denmark, Estonia, Spain, Finland, France, United Kingdom, Greece, Hungary, Ireland, Iceland, Italy, Liechtenstein, Lithuania, Latvia, Monaco, Netherlands, Poland, Portugal, Romania, Sweden, Slovenia, Slovakia, World Intellectual Property Organization (WIPO) |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Compulsory licence on dolutegravir in Colombia
https://www.statnews.com/wp-content/uploads/2024/04/NC_534_Licencia_obligatoria_aceptada.pdfPatent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Tenofovir alafenamide hemifumarate (TAF)
Expiry date: 2032-08-15 A hemifumarate form of tenofovir alafenamide, and antiviral therapy using tenofovir alafenamide hemifurnarate (e.g., anti-HTV and anti-HBV therapies). |
WO2013025788 | Salt | Gilead Sciences, Inc | No | US FDA |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Morocco, Moldova, Republic of, Mexico, Peru, Botswana, Ghana, Gambia (the), Kenya, Liberia, Lesotho, Malawi, Mozambique, Namibia, Rwanda, Sudan, Sierra Leone, Eswatini, Tanzania, United Republic of, Uganda, Zambia, Zimbabwe, Armenia, Azerbaijan, Belarus, Kyrgyzstan, Kazakhstan, Tajikistan, Turkmenistan, Viet Nam, Benin, Cameroon, Burkina Faso, Chad, Guinea-Bissau, Mali, Senegal, Congo, Guinea, Gabon, Niger, Equatorial Guinea, Mauritania, Togo, Côte d'Ivoire, Central African Republic, Bolivia (Plurinational State of), Philippines, South Africa, Ukraine, Brazil, El Salvador, Montenegro, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Serbia | United States of America, Australia, Canada, Chile, Costa Rica, Hong Kong, Israel, Japan, Korea, Republic of, New Zealand, Singapore, Taiwan, Province of China, Uruguay, Russian Federation, Denmark, Panama, Croatia, Cyprus, Bahamas, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Monaco, Portugal, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Romania, Latvia, Lithuania, Slovenia |
| Filed | China, Ecuador, India, Thailand, Venezuela (Bolivarian Republic of), Türkiye, North Macedonia, Albania, Serbia, Egypt | Hong Kong, Denmark, Bahrain, Kuwait, Qatar, Saudi Arabia, Oman, United Arab Emirates, Croatia, Cyprus, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Monaco, Portugal, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Romania, Latvia, Lithuania, Slovenia |
| Not in force | World Intellectual Property Organization (WIPO), Argentina, China, Colombia, Indonesia, Pakistan, Paraguay, Brazil, Montenegro, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Serbia | World Intellectual Property Organization (WIPO), Hong Kong, Israel, Japan, New Zealand, Denmark, Croatia, Cyprus, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Monaco, Portugal, Ireland, Finland, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Romania, Latvia, Lithuania, Slovenia |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
TAF manufacturing process
Expiry date: 2032-10-03 Methods for isolating 9-{(R)-2-[((S)-{[(S)-l - (isopropoxycarbonyl)ethyl]amino}phenoxyphosphinyl)methoxy]propyl}adenine:'' (compound 16): a method for preparing, in high diastereomeric purity, intermediate compounds 13 and 15: method for preparing intermediate compound 12: 9-{(R)-2-[((S)-{[(S)-l - (isopropoxycarbonyl)ethyl]amino}phenoxyphosphinyl)methoxy]propyl}adenine has anti-viral properties. |
WO2013052094 | Process | Gilead Sciences, Inc | No | MPP licence |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | China, Colombia, Mexico, Armenia, Azerbaijan, Belarus, Kyrgyzstan, Kazakhstan, Tajikistan, Turkmenistan, Türkiye, Brazil, Montenegro, India, Bolivia (Plurinational State of) | Australia, Canada, Hong Kong, Japan, Korea, Republic of, Taiwan, Province of China, United States of America, Russian Federation, Austria, Belgium, Switzerland, Czechia, Germany, Spain, France, United Kingdom, Greece, Ireland, Italy, Liechtenstein, Netherlands, Norway, Poland, Portugal, Sweden, Slovenia, Slovakia, New Zealand, Israel, Bahamas, Macao |
| Filed | China | Hong Kong, Korea, Republic of |
| Not in force | Argentina, Peru, Albania, North Macedonia, Serbia, Türkiye, World Intellectual Property Organization (WIPO), Brazil, Bosnia and Herzegovina, Montenegro, Ecuador, El Salvador, Pakistan, Egypt, Paraguay, Venezuela (Bolivarian Republic of) | Chile, Costa Rica, Japan, Uruguay, Bulgaria, Cyprus, Czechia, Denmark, Estonia, Finland, Greece, Croatia, Hungary, Iceland, Lithuania, Luxembourg, Latvia, Monaco, Malta, Norway, Poland, Romania, Slovenia, Slovakia, San Marino, World Intellectual Property Organization (WIPO), Kuwait, United Arab Emirates, Bahrain, Saudi Arabia, Oman, Qatar, Panama |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Dolutegravir/Cabotegravir intermediates production processes & Intermediates
Expiry date: 2029-12-09 Processes are provided which create an aldehyde methylene, or hydrated or hemiacetal methylene attached to a heteroatom of a 6 membered ring without going through an olefinic group and without the necessity of using an osmium reagent. In particular, a compound of formula (I) can be produced from (II) and avoid the use of an allyl amine: (formulae I and II) where R, P 1 P3, R3 and Rx are as described herein. |
WO2010068262 | Intermediate(s), Process | Sionogi & Co., Ltd, Viiv Healthcare Company | No | MPP Licence |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | China, India | Japan, Korea, Republic of, Singapore, Taiwan, Province of China, United States of America, Belgium, Germany, France, Netherlands, Switzerland, United Kingdom, Italy, Liechtenstein, Spain, Portugal |
| Filed | ||
| Not in force | China, World Intellectual Property Organization (WIPO), Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Serbia | World Intellectual Property Organization (WIPO), Luxembourg, Sweden, Austria, Greece, Denmark, Monaco, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania, Slovenia |
Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Dolutegravir salts, their crystals & process
Expiry date: 2029-12-08 A synthesis approach providing an early ring attachment via a bromination to compound l-l yielding compound II-Il, whereby a final product such as AA can be synthesized. In particular, the 2,4-difluorophenyl-containing sidechain is attached before creation of the additional ring Q. |
WO2010068253 | Process, Salt | Glaxosmithkline Llc, Johns, Brian, Alvin, Shionogi & Co., Ltd, Taoda, Yoshiyuki, Yoshida, Hiroshi | Yes | US FDA |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | China, Mexico, Brazil, Indonesia, India | United States of America, Australia, Canada, Switzerland, Germany, Spain, France, United Kingdom, Ireland, Italy, Liechtenstein, Japan, Korea, Republic of, Russian Federation, Singapore, Taiwan, Province of China |
| Filed | Canada | |
| Not in force | World Intellectual Property Organization (WIPO), North Macedonia, Türkiye, India | World Intellectual Property Organization (WIPO), Austria, Belgium, Bulgaria, Switzerland, Cyprus, Czechia, Germany, Denmark, Estonia, Spain, Finland, France, United Kingdom, Greece, Croatia, Hungary, Ireland, Iceland, Italy, Liechtenstein, Lithuania, Luxembourg, Latvia, Monaco, Malta, Netherlands, Norway, Poland, Portugal, Romania, Sweden, Slovenia, Slovakia, San Marino, Hong Kong |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Cabotegravir prodrugs & Cabotegravir and Dolutegravir intermediates and processes
Expiry date: 2029-07-23 The present invention features compounds that are prodrugs of HIV integrase inhibitors and therefore are useful in the delivery of compounds for the inhibition of HIV replication, the prevention and/or treatment of infection by HIV, and in the treatment of AIDS and/or ARC. |
WO2010011814 | Intermediate(s), Process | Glaxosmithkline Llc, Shionogi & Co., Ltd, Viiv Healthcare Company | Yes | MPP Licence |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | China, India | Belgium, Germany, France, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Liechtenstein, Spain, Portugal, Japan, Korea, Republic of, Singapore, United States of America |
| Filed | ||
| Not in force | Türkiye, North Macedonia, Bosnia and Herzegovina, World Intellectual Property Organization (WIPO), Albania, Serbia | Belgium, France, Luxembourg, Netherlands, Switzerland, Sweden, Austria, Liechtenstein, Greece, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania, Slovenia, World Intellectual Property Organization (WIPO) |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Dolutegravir in combination with lamivudine (3TC)
Expiry date: 2031-01-24 The present disclosure relates to combinations of compounds comprising HIV integrase inhibitors and other therapeutic agents. Such combinations may be useful in the inhibition of HIV-1 or potentially the inhibition of HIV replication, or for the prevention and/or treatment of infection by HIV, or in the treatment of AIDS and/or ARC. |
CA3003988 | Combination | Viiv Healthcare Company | Yes | MPP licence |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Turkmenistan, Belarus, Tajikistan, Kazakhstan, Azerbaijan, Kyrgyzstan, Armenia, Türkiye, North Macedonia, Albania, Bosnia and Herzegovina, Montenegro, Serbia, Mexico, Philippines, Malaysia | United States of America, Canada, Australia, Russian Federation, Belgium, Germany, France, Luxembourg, Netherlands, Switzerland, United Kingdom, Sweden, Italy, Austria, Liechtenstein, Greece, Spain, Denmark, Monaco, Portugal, Ireland, Finland, Cyprus, Bulgaria, Czechia, Estonia, Slovakia, Hungary, Poland, Iceland, Malta, Norway, San Marino, Croatia, Romania, Latvia, Lithuania, Slovenia, Israel |
| Filed | Singapore | |
| Not in force | Moldova, Republic of, Dominican Republic | United States of America, Australia |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Dolutegravir or cabotegravir in combination with ABC, 3TC or RPV
Expiry date: 2031-01-24 The present invention relates to combinations of compounds comprising HIV integrase inhibitors and other therapeutic agents. Such combinations are useful in the inhibition of HIV replication, the prevention and/or treatment of infection by HIV, and in the treatment of AIDS and/or ARC. |
WO2011094150 | Combination | Glaxosmithkline Llc, Underwood, Mark Richard | Yes | MPP licence |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | Philippines, Viet Nam, Malaysia | Hong Kong |
| Filed | Algeria, Egypt, Philippines, Viet Nam, Malaysia | Oman |
| Not in force | Ecuador, Libya, World Intellectual Property Organization (WIPO), Brazil, Tajikistan, Belarus, Azerbaijan, Moldova, Republic of, Turkmenistan, Armenia, Kyrgyzstan, Kazakhstan | Costa Rica, Hong Kong, World Intellectual Property Organization (WIPO), Russian Federation |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/MPP licence on paediatric formulations of dolutegravir (DTG)
https://medicinespatentpool.org/licence-post/dolutegravir-paediatrics-dtg/MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations in AZ, BY, KZ and MY
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg-umics/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Dolutegravir intermediates, their crystalline forms and processes
Expiry date: 2030-03-26 |
WO2010110409 | Intermediate(s), Process | Yes | MPP licence |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | China, India | Liechtenstein, Italy, Belgium, United Kingdom, Netherlands, Switzerland, Spain, Austria, Romania, France, Ireland, Germany, Japan, Korea, Republic of, Singapore, Taiwan, Province of China, United States of America |
| Filed | ||
| Not in force | Albania, Serbia, Bosnia and Herzegovina, Montenegro, Türkiye, North Macedonia, World Intellectual Property Organization (WIPO) | Liechtenstein, Italy, Norway, Malta, Denmark, Belgium, United Kingdom, Greece, Netherlands, Hungary, Croatia, Switzerland, Spain, San Marino, Slovenia, Austria, Romania, Iceland, Cyprus, Finland, France, Bulgaria, Slovakia, Poland, Latvia, Ireland, Estonia, Germany, Luxembourg, Portugal, Czechia, Lithuania, Monaco, Sweden, World Intellectual Property Organization (WIPO) |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/Patent informations
| Patent description | Representative patent | Categories | Patent holder | Licence with MPP | Patent source |
|---|---|---|---|---|---|
|
Dolutegravir or cabotegravir intermediates and processes
Expiry date: 2031-08-04 |
WO2012018065 | Intermediate(s), Process | Yes | MPP licence |
Patent status
| Patent status/countries | Low, Low- middle and upper-middle | High income |
|---|---|---|
| Granted | China, Albania, Serbia, Türkiye, North Macedonia, India, Indonesia | Liechtenstein, Italy, Norway, Malta, Denmark, Belgium, United Kingdom, Greece, Netherlands, Hungary, Croatia, Switzerland, Spain, San Marino, Slovenia, Austria, Romania, Iceland, Cyprus, Finland, France, Bulgaria, Slovakia, Poland, Latvia, Ireland, Estonia, Germany, Luxembourg, Portugal, Czechia, Lithuania, Monaco, Sweden, Japan, Singapore, Taiwan, Province of China, United States of America |
| Filed | Brazil | |
| Not in force | World Intellectual Property Organization (WIPO), Albania, Serbia, Bosnia and Herzegovina, Montenegro, Türkiye, North Macedonia, India | World Intellectual Property Organization (WIPO), Norway, Malta, Denmark, Greece, Hungary, Croatia, San Marino, Slovenia, Austria, Romania, Iceland, Cyprus, Finland, Bulgaria, Slovakia, Poland, Latvia, Ireland, Estonia, Luxembourg, Czechia, Lithuania, Monaco, Sweden, United States of America |
MPP Licence(s)
MPP licence on adult formulations of dolutegravir (DTG) and DTG/ABC combinations
https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg/Supporting material
Publications
<p>Qingxin Mu, Jesse Yu, Lisa A. McConnachie, John C. Kraft, Yu Gao, Gaurav K. Gulati & Rodney J. Y. Ho (2018)</p><p><a href="https://doi.org/10.1080/1061186X.2017.1419363" rel="noopener noreferrer" target="_blank">Translation of combination nanodrugs into nanomedicines: lessons learned and future outlook</a> </p><p>Journal of Drug Targeting, 26:5-6, 435-447, DOI: 10.1080/1061186X.2017.1419363</p>
The concept of nanomedicine is not new. For instance, some nanocrystals and colloidal drug molecules are marketed that improve pharmacokinetic characteristics of single-agent therapeutics. For the past two decades, the number of research publications on single-agent nanoformulations has grown exponentially. However, formulations advancing to pre-clinical and clinical evaluations that lead to therapeutic products has been limited. Chronic diseases such as cancer and HIV/AIDS require drug combinations, not single agents, for durable therapeutic responses. Therefore, development and clinical translation of drug combination nanoformulations could play a significant role in improving human health. Successful translation of promising concepts into pre-clinical and clinical studies requires early considerations of the physical compatibility, pharmacological synergy, as well as pharmaceutical characteristics (e.g. stability, scalability and pharmacokinetics). With this approach and robust manufacturing processes in place, some drug-combination nanoparticles have progressed to non-human primate and human studies. In this article, we discuss the rationale and role of drug-combination nanoparticles, the pre-clinical and clinical research progress made to date and the key challenges for successful clinical translation. Finally, we offer insight to accelerate clinical translation through leveraging robust nanoplatform technologies to enable implementation of personalised and precision medicine.
<p>John C. Kraft, Lisa A. McConnachie, Josefin Koehn, Loren Kinman, Jianguo Sun, Ann C. Collier, Carol Collins, Danny D. Shen, Rodney J.Y. Ho,</p><p><a href="https://www.sciencedirect.com/science/article/abs/pii/S0168365918300634?via%3Dihub" rel="noopener noreferrer" target="_blank">Mechanism-based pharmacokinetic (MBPK) models describe the complex plasma kinetics of three antiretrovirals delivered by a long-acting anti-HIV drug combination nanoparticle formulation</a></p><p>Journal of Controlled Release, Volume 275, 2018, Pages 229-241, ISSN 0168-3659, https://doi.org/10.1016/j.jconrel.2018.02.003.</p>
Existing oral antiretroviral (ARV) agents have been shown in human studies to exhibit limited lymph node penetration and lymphatic drug insufficiency. As lymph nodes are a reservoir of HIV, it is critical to deliver and sustain effective levels of ARV combinations in these tissues. To overcome lymph node drug insufficiency of oral combination ARV therapy (cART), we developed and reported a long-acting and lymphocyte-targeting injectable that contains three ARVs—hydrophobic lopinavir (LPV) and ritonavir (RTV), and hydrophilic tenofovir (TFV)—stabilized by lipid excipients in a nanosuspension. A single subcutaneous (SC) injection of this first-generation formulation of drug combination nanoparticles (DcNPs), named TLC-ART101, provided persistent ARV levels in macaque lymph node mononuclear cells (LNMCs) for at least 1 week, and in peripheral blood mononuclear cells (PBMCs) and plasma for at least 2 weeks, demonstrating long-acting pharmacokinetics for all three drugs. In addition, the lymphocyte-targeting properties of this formulation were demonstrated by the consistently higher intracellular drug concentrations in LNMCs and PBMCs versus those in plasma. To provide insights into the complex mechanisms of absorption and disposition of TLC-ART101, we constructed novel mechanism-based pharmacokinetic (MBPK) models. Based upon plasma PK data obtained after single administration of TLC-ART101 (DcNPs) and a solution formulation of free triple-ARVs, the models feature uptake from the SC injection site that respectively routes free and nanoparticle-associated ARVs via the blood vasculature and lymphatics, and their eventual distribution into and clearance from the systemic circulation. The models provided simultaneous description of the complex long-acting plasma and lymphatic PK profiles for all three ARVs in TLC-ART101. The long-acting PK characteristics of the three drugs in TLC-ART101 were likely due to a combination of mechanisms including: (1) DcNPs undergoing preferential lymphatic uptake from the subcutaneous space, (2) retention in nodes during lymphatic first-pass, (3) subsequent slow release of ARVs into blood circulation, and (4) limited extravasation of DcNP-associated ARVs that resulted in longer persistence in the circulation.
Keywords: Mechanism-based pharmacokinetic modeling; Long-acting; Antiretrovirals; HIV drug combination treatment; Lymphatic targeted drug delivery; Lymphatic drug insufficiency
<p>Mu Q, Yu J, Griffin JI, Wu Y, Zhu L, McConnachie LA, et al. (2020) </p><p><a href="https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0228557" rel="noopener noreferrer" target="_blank">Novel drug combination nanoparticles exhibit enhanced plasma exposure and dose-responsive effects on eliminating breast cancer lung metastasis</a>. </p><p>PLoSONE 15(3): e0228557. </p><p>https://doi.org/10.1371/journal.pone.0228557</p>
Early diagnosis along with new drugs targeted to cancer receptors and immunocheckpoints have improved breast cancer survival. However, full remission remains elusive for metastatic breast cancer due to dose-limiting toxicities of heavily used, highly potent drug combinations such as gemcitabine and paclitaxel. Therefore, novel strategies that lower the effective dose and improve safety margins could enhance the effect of these drug combinations. To this end, we developed and evaluated a novel drug combination of gemcitabine and paclitaxel (GT). Leveraging a simple and scalable drug-combination nanoparticle platform (DcNP), we successfully prepared an injectable GT combination in DcNP (GT DcNP). Compared to a Cremophor EL/ethanol assisted drug suspension in buffer (CrEL), GT DcNP exhibits about 56-fold and 8.6-fold increases in plasma drug exposure (area under the curve, AUC) and apparent half-life of gemcitabine respectively, and a 2.9-fold increase of AUC for paclitaxel. Using 4T1 as a syngeneic model for breast cancer metastasis, we found that a single GT (20/2 mg/kg) dose in DcNP nearly eliminated colonization in the lungs. This effect was not achievable by a CrEL drug combination at a 5-fold higher dose (i.e., 100/10 mg/kg GT). A dose-response study indicates that GT DcNP provided a therapeutic index of ~15.8. Collectively, these data suggest that GT DcNP could be effective against advancing metastatic breast cancer with a margin of safety. As the DcNP formulation is intentionally designed to be simple, scalable, and long-acting, it may be suitable for clinical development to find effective treatment against metastatic breast cancer.
<p>Koehn, Josefina; Iwamoto, Jennifer F.a; Kraft, John C.a; McConnachie, Lisa A.a; Collier, Ann C.b,c; Ho, Rodney J.Y.a,c,d </p><p><a href="https://journals.lww.com/aidsonline/Fulltext/2018/11130/Extended_cell_and_plasma_drug_levels_after_one.3.aspx" rel="noopener noreferrer" target="_blank">Extended cell and plasma drug levels after one dose of a three-in-one nanosuspension containing lopinavir, efavirenz, and tenofovir in nonhuman primates</a></p><p>AIDS: November 13, 2018 - Volume 32 - Issue 17 - p 2463-2467</p><p>doi: 10.1097/QAD.0000000000001969 </p>
Objective:
To characterize a drug-combination nanoparticle (DcNP) containing water-insoluble lopinavir (LPV) and efavirenz (EFV), and water-soluble tenofovir (TFV), for its potential as a long-acting combination HIV treatment.
Design:
Three HIV drugs (LPV, EFV, TFV) with well established efficacy and safety were coformulated into a single DcNP suspension. Two macaques were administered one subcutaneous injection and drug concentrations in plasma and mononuclear cells (in peripheral blood and lymph nodes) were analyzed over 2 weeks. Pharmacokinetic parameters and cell-to-plasma relationships of LPV, EFV, and TFV were determined.
Results:
This three-in-one nanoformulation provided extended concentrations of all drugs in lymph node cells that were 57- to 228-fold higher than those in plasma. Levels of all three drugs in peripheral blood mononuclear cells persisted for 2 weeks at levels equal to or higher than those in plasma.
Conclusion:
With long-acting characteristics and higher drug penetration/persistence in cells, this three-in-one DcNP may enhance therapeutic efficacy of these well studied HIV drugs due to colocalization and targeting of this three-drug combination to HIV host cells.
<p>Yu Gao, John C. Kraft, Danni Yu, Rodney J.Y. Ho,</p><p><a href="https://www.sciencedirect.com/science/article/abs/pii/S0939641118301577?via%3Dihub" rel="noopener noreferrer" target="_blank">Recent developments of nanotherapeutics for targeted and long-acting, combination HIV chemotherapy</a>,</p><p>European Journal of Pharmaceutics and Biopharmaceutics,</p><p>Volume 138, 2019, Pages 75-91, ISSN 0939-6411,</p><p>https://doi.org/10.1016/j.ejpb.2018.04.014.</p>
Combination antiretroviral therapy (cART) given orally has transformed HIV from a terminal illness to a manageable chronic disease. Yet despite the recent development of newer and more potent drugs for cART and suppression of virus in blood to undetectable levels, residual virus remains in tissues. Upon stopping cART, virus rebounds and progresses to AIDS. Current oral cART regimens have several drawbacks including (1) challenges in patient adherence due to pill fatigue or side-effects, (2) the requirement of life-long daily drug intake, and (3) limited penetration and retention in cells within lymph nodes. Appropriately designed injectable nano-drug combinations that are long-acting and retained in HIV susceptible cells within lymph nodes may address these challenges. While a number of nanomaterials have been investigated for delivery of HIV drugs and drug combinations, key challenges involve developing and scaling delivery systems that provide a drug combination targeted to HIV host cells and tissues where residual virus persists. With validation of the drug-insufficiency hypothesis in lymph nodes, progress has been made in the development of drug combination nanoparticles that are long-acting and targeted to lymph nodes and cells. Unique drug combination nanoparticles (DcNPs) composed of three HIV drugs—lopinavir, ritonavir, and tenofovir—have been shown to provide enhanced drug levels in lymph nodes; and elevated drug-combination levels in HIV-host cells in the blood and plasma for two weeks. This review summarizes the progress in the development of nanoparticle-based drug delivery systems for HIV therapy. It discusses how injectable nanocarriers may be designed to enable delivery of drug combinations that are long-lasting and target-selective in physiological contexts (in vivo) to provide safe and effective use. Consistent drug combination exposure in the sites of residual HIV in tissues and cells may overcome drug insufficiency observed in patients on oral cART.
Keywords: Nanomedicine; Long-acting; Targeted; Drug combination; HIV/AIDS
<p>Yu, J., Mu, Q., Perazzolo, S. et al. </p><p><a href="https://link.springer.com/article/10.1007%2Fs11095-020-02888-8#citeas" rel="noopener noreferrer" target="_blank">Novel Long-Acting Drug Combination Nanoparticles Composed of Gemcitabine and Paclitaxel Enhance Localization of Both Drugs in Metastatic Breast Cancer Nodules. </a></p><p>Pharm Res 37, 197 (2020). https://doi.org/10.1007/s11095-020-02888-8</p>
Purpose
To develop drug-combination nanoparticles (DcNPs) composed of hydrophilic gemcitabine (G) and hydrophobic paclitaxel (T) and deliver both drugs to metastatic cancer cells.
Methods
GT DcNPs were evaluated based on particle size and drug association efficiency (AE%). The effect of DcNP on GT plasma time-course and tissue distribution was characterized in mice and a pharmacokinetic model was developed. A GT distribution study into cancer nodules (derived from 4 T1 cells) was performed.
Results
An optimized GT DcNP composition (d = 59.2 nm ±9.2 nm) was found to be suitable for IV formulation. Plasma exposure of G and T were enhanced 61-fold and 3.8-fold when given in DcNP form compared to the conventional formulation, respectively. Mechanism based pharmacokinetic modeling and simulation show that both G and T remain highly associated to DcNPs in vivo (G: 98%, T:75%). GT DcNPs have minimal distribution to healthy organs with selective distribution and retention in tumor burdened tissue. Tumor bearing lungs had a 5-fold higher tissue-to-plasma ratio of gemcitabine in GT DcNPs compared to healthy lungs.
Conclusions
DcNPs can deliver hydrophilic G and hydrophobic T together to cancer nodules and produce long acting exposure, likely due to stable GT association to DcNPs in vivo.
<p>Kraft, John C.a; McConnachie, Lisa A.a; Koehn, Josefina; Kinman, Lorena; Collins, Carola; Shen, Danny D.a; Collier, Ann C.b,c; Ho, Rodney J.Y.a,c,d <a href="https://journals.lww.com/aidsonline/Fulltext/2017/03270/Long_acting_combination_anti_HIV_drug_suspension.4.aspx" rel="noopener noreferrer" target="_blank">Long-acting combination anti-HIV drug suspension enhances and sustains higher drug levels in lymph node cells than in blood cells and plasma,</a> </p><p>AIDS: March 27, 2017 - Volume 31 - Issue 6 - p 765-770</p><p>doi: 10.1097/QAD.0000000000001405 </p>
Objective:
The aim of the present study was to determine whether a combination of anti-HIV drugs – tenofovir (TFV), lopinavir (LPV) and ritonavir (RTV) – in a lipid-stabilized nanosuspension (called TLC-ART101) could enhance and sustain intracellular drug levels and exposures in lymph node and blood cells above those in plasma.
Design:
Four macaques were given a single dose of TLC-ART101 subcutaneously. Drug concentrations in plasma and mononuclear cells of the blood (PBMCs) and lymph nodes (LNMCs) were analysed using a validated combination LC-MS/MS assay.
Results:
For the two active drugs (TFV, LPV), plasma and PBMC intracellular drug levels persisted for over 2 weeks; PBMC drug exposures were three- to four-fold higher than those in plasma. Apparent terminal half-lives (t1/2) of TFV and LPV were 65.3 and 476.9 h in plasma, and 169.1 and 151.2 h in PBMCs. At 24 and 192 h, TFV and LPV drug levels in LNMCs were up to 79-fold higher than those in PBMCs. Analysis of PBMC intracellular TFV and its active metabolite TFV-diphosphate (TFV-DP) indicated that intracellular exposures of total TFV and TFV-DP were markedly higher and persisted longer than in humans and macaques dosed with oral TFV prodrugs, tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide (TAF).
Conclusions:
A simple, scalable three-drug combination, lipid-stabilized nanosuspension exhibited persistent drug levels in cells of lymph nodes and the blood (HIV host cells) and in plasma. With appropriate dose adjustment, TLC-ART101 may be a useful HIV treatment with a potential to impact residual virus in lymph nodes.
<p>John C. Kraft, Piper M. Treuting & Rodney J. Y. Ho (2018) </p><p><a href="https://www.tandfonline.com/doi/full/10.1080/1061186X.2018.1433681" rel="noopener noreferrer" target="_blank">Indocyanine green nanoparticles undergo selective lymphatic uptake, distribution and retention and enable detailed mapping of lymph vessels, nodes and abnormalities</a></p><p>Journal of Drug Targeting, 26:5-6, 494-504, DOI: 10.1080/1061186X.2018.1433681</p>
The distributed network of lymph vessels and nodes in the body, with its complex architecture and physiology, presents a major challenge for whole-body lymphatic-targeted drug delivery. To gather physiological and pathological information of the lymphatics, near-infrared (NIR) fluorescence imaging of NIR fluorophores is used in clinical practice due to its tissue-penetrating optical radiation (700–900 nm) that safely provides real-time high-resolution in vivo images. However, indocyanine green (ICG), a common clinical NIR fluorophore, is unstable in aqueous environments and under light exposure, and its poor lymphatic distribution and retention limits its use as a NIR lymphatic tracer. To address this, we investigated in mice the distribution pathways of a novel nanoparticle formulation that stabilises ICG and is optimised for lymphatic drug delivery. From the subcutaneous space, ICG particles provided selective lymphatic uptake, lymph vessel and node retention, and extensive first-pass lymphatic distribution of ICG, enabling 0.2 mm and 5–10 cell resolution of lymph vessels, and high signal-to-background ratios for lymphatic vessel and node networks. Soluble (free) ICG readily dissipated from lymph vessels local to the injection site and absorbed into the blood. These unique characteristics of ICG particles could enable mechanistic studies of the lymphatics and diagnosis of lymphatic abnormalities.
<p><a href="https://pubmed.ncbi.nlm.nih.gov/34673093/" rel="noopener noreferrer" target="_blank">Perazzolo S, Shireman LM, Shen DD, Ho RJY. Physiologically Based Pharmacokinetic Modeling of 3 HIV Drugs in Combination and the Role of Lymphatic System after Subcutaneous Dosing. Part 1: Model for the Free-drug Mixture. J Pharm Sci. 2021 Oct 18:S0022-3549(21)00547-5. doi: 10.1016/j.xphs.2021.10.007. Epub ahead of print. PMID: 34673093.</a></p>
Drug-combination nanoparticles (DcNP) is a nano-formulation of multiple HIV drugs in one injectable. DcNP demonstrated long-acting pharmacokinetics (PK) for all drugs in the blood and lymphatic system of nonhuman primates (NHP). Long-acting is due to stably circulating DcNP and a depot in the lymphatic system during subcutaneous absorption. Because the PK of each drug in DcNP evolves through two species, i.e., drugs that dissociate from DcNP and drugs retained in DcNP (Part 2, presented separately), we describe here a physiologically based PK model of the nanoparticle-free drugs featuring the role of the lymphatic system. The free drug model was built using subcutaneous injections of suspended lopinavir-ritonavir-tenofovir in NHP and validated by external experiments. The model, for the first time, introduces the lymphatic network as part of a whole-body PBPK system and singles out major lymphatic regions: cervical, axillary, hilar, mesenteric, and inguinal nodes. Although the scope of the free-drug modeling was to support the construction of the nanoparticle model (Part 2), such a detailed/regionalized description of the lymphatic system and mononuclear cells represent an unprecedented level of prediction that renders the free drug model extendible to other small-drug molecules targeting the lymphatic system at both the regional and cellular level.
<p><a href="https://pubmed.ncbi.nlm.nih.gov/34673093/" rel="noopener noreferrer" target="_blank">Perazzolo S, Shen DD, Ho RJY. Physiologically Based Pharmacokinetic Modeling of 3 HIV Drugs in Combination and the Role of Lymphatic System after Subcutaneous Dosing. Part 2: Model for the Drug-combination Nanoparticles. J Pharm Sci. 2021 Oct 18:S0022-3549(21)00552-9. doi: 10.1016/j.xphs.2021.10.009. Epub ahead of print. PMID: 34673094.</a></p>
Drug-combination nanoparticles (DcNP) is a nano-formulation of multiple HIV drugs in one injectable. DcNP demonstrated long-acting pharmacokinetics (PK) for all drugs in the blood and lymphatic system of nonhuman primates (NHP). Long-acting is due to stably circulating DcNP and a depot in the lymphatic system during subcutaneous absorption. Because the PK of each drug in DcNP evolves through two species, i.e., drugs that dissociate from DcNP and drugs retained in DcNP (Part 2, presented separately), we describe here a physiologically based PK model of the nanoparticle-free drugs featuring the role of the lymphatic system. The free drug model was built using subcutaneous injections of suspended lopinavir-ritonavir-tenofovir in NHP and validated by external experiments. The model, for the first time, introduces the lymphatic network as part of a whole-body PBPK system and singles out major lymphatic regions: cervical, axillary, hilar, mesenteric, and inguinal nodes. Although the scope of the free-drug modeling was to support the construction of the nanoparticle model (Part 2), such a detailed/regionalized description of the lymphatic system and mononuclear cells represent an unprecedented level of prediction that renders the free drug model extendible to other small-drug molecules targeting the lymphatic system at both the regional and cellular level.
<p>A novel formulation enabled transformation of 3-HIV drugs tenofovir–lamivudine–dolutegravir from short-acting to long-acting all-in-one injectable. Perazzolo, Simonea; Stephen, Zachary R.a; Eguchi, Masaa; Xu, Xiaolina; Delle Fratte, Rachelea; Collier, Ann C.b; Melvin, Ann J.c; Ho, Rodney J.Y.a,d. </p><p>AIDS 37(14):p 2131-2136, November 15, 2023. | DOI: 10.1097/QAD.0000000000003706 </p><p><br></p><p>https://journals.lww.com/aidsonline/abstract/2023/11150/a_novel_formulation_enabled_transformation_of.6.aspx</p>
Objective:
To develop an injectable dosage form of the daily oral HIV drugs, tenofovir (T), lamivudine (L), and dolutegravir (D), creating a single, complete, all-in-one TLD 3-drug-combination that demonstrates long-acting pharmacokinetics.
Design:
Using drug-combination-nanoparticle (DcNP) technology to stabilize multiple HIV drugs, the 3-HIV drugs TLD, with disparate physical-chemical properties, are stabilized and assembled with lipid-excipients to form TLD-in-DcNP. TLD-in-DcNP is verified to be stable and suitable for subcutaneous administration. To characterize the plasma time-courses and PBMC concentrations for all 3 drugs, single subcutaneous injections of TLD-in-DcNP were given to nonhuman primates (NHP, M. nemestrina).
Results:
Following single-dose TLD-in-DcNP, all drugs exhibited long-acting profiles in NHP plasma with levels that persisted for 4 weeks above predicted viral-effective concentrations for TLD in combination. Times-to-peak were within 24 hr in all NHP for all drugs. Compared to a free-soluble TLD, TLD-in-DcNP provided exposure enhancement and extended duration 7.0-, 2.1-, and 20-fold as AUC boost and 10-, 8.3-, and 5.9-fold as half-life extension. Additionally, DcNP may provide more drug exposure in cells than plasma with PBMC-to-plasma drug ratios exceeding one, suggesting cell-targeted drug-combination delivery.
Conclusions:
This study confirms that TLD with disparate properties can be made stable by DcNP to enable TLD concentrations of 4 weeks in NHP. Study results highlighted the potential of TLD-in-DcNP as a convenient all-in-one, complete HIV long-acting product for clinical development.
Additional documents
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Illustrations

TLD nanoparticle
None