
|
Developed by 

|
Supported by 


|
Cloudbreak (r)
Based on public informationDeveloper(s)

|
Cidara Therapeutics https://www.cidara.com/United States of America Cidara Therapeutics has developed a drug development pipeline platform called CloudBreak which is based on API conjugation with antibody fragment conjugate. This Drug Fc conjugate is a targeted immunotherapy that inhibits specific disease targets while simultaneously engaging the immune system. Cidara's portfolio includes novel therapeutics targeting viral infections and solid tumors. |
Sponsor(s)
|
No sponsor indicated |
Partnerships

|
Janseen Pharmaceuticals https://www.janssen.com |
Technology information
Type of technology
Peptide of Human Antibody Fragment (Fc) coupled with drug molecule, Monoclonal antibodies and antibody drug conjugates
Administration route
Subcutaneous, Intramuscular, Intravenous
Development state and regulatory approval
Temsavir
Pre-clinical
Not provided
Description
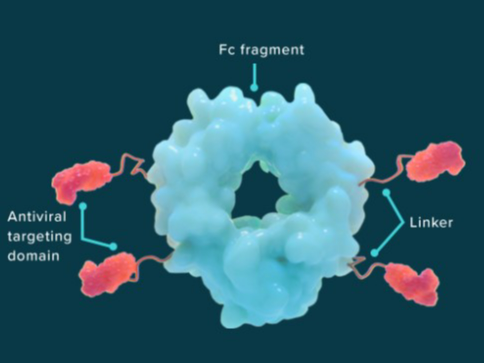
Cloudbreak (r) is a Drug Fc Conjugate that acts as a single-molecule cocktail by coupling targeted small molecules and peptides to a human antibody fragment (Fc). These conjugates bind to the target receptors for an extended period while simultaneously engaging with the immune system of the human body, allowing them to both treat and prevent disease. antibody drug conjugates like this have the potential to carry multiple drug molecules (payloads).
Technology highlight
• Long half-life, similar to monoclonal antibody • Ability to target cryptic sites & has small molecule binding pockets • Targeted pharmacological action with low systematic exposure • Multivalent target engagement of DFC increases potency of API and reduces resistance potential • Extracellular targeted action
Technology main components
Drug -Fc Conjugate cocktail consists of (i) API, (ii) peptide fusions, and (iii) a human antibody fragment specific to the targeted disease (Fc MOIETY).
Information on the raw materials sourcing, availability and anticipated price
Not provided
Delivery device(s)
No delivery device
APIs compatibility profile
API desired features
Water-soluble molecules
Water-insoluble molecules
Small molecules
The DFC technology targets small molecules like Influenza neuroaminidase inhibitors (such as oseltamivir, zanamivir, peramivir, and laninamivir), CD73 inhibitors, CCR antagonists and GP120 inhibitors (such as temsavir).
Additional solubility data
Not provided
Additional stability data
Not provided
API loading: Maximum drug quantity to be loaded
Not provided
API co-administration
Not provided
LogP
Not provided
Scale-up and manufacturing prospects
Scale-up prospects
WuXi XDC partnered with Cidara Therapeutics to manufacture DFC formulations. WuXi recently established a new manufacturing facility with the capacity to produce 200-2000 litres of DFC formulation per batch.
Tentative equipment list for manufacturing
Not provided
Manufacturing
Manufacturing of DFC formulation has low COGS.
Specific analytical instrument required for characterization of formulation
HPLC analytical procedure is used for the gross content and assay of reconstituted solution tests in the drug product specification
Clinical trials
CD388.SQ.2.02
Identifier
NCT05523089
Link
https://clinicaltrials.gov/study/NCT05523089
Phase
Phase II
Status
Completed
Sponsor
Cidara Therapeutics Inc.
More details
The purpose of this study is to evaluate the preventative antiviral activity of CD388, as compared to saline placebo, when administered as a single dose to healthy adult participants in a human viral challenge model of influenza.
Purpose
The Effectiveness of CD388 to Prevent Flu in an Influenza Challenge Model in Healthy Adults
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-09-09
Anticipated Date of Last Follow-up
2024-09-04
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2023-07-17
Actual Completion Date
2023-07-17
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: 1. Written informed consent signed and dated by the participant and the PI/investigator obtained before any assessment is performed. 2. Adult male or female aged between 18 and 55 years old, inclusive, on the day prior to signing the consent form. 3. A total body weight ≥50 kilograms (kg) and body mass index (BMI) ≥18 kg/meter squared (m\^2) and ≤35kg/m\^2. 4. In good health with no history, or current evidence, of clinically significant medical conditions, and no clinically significant test abnormalities that will interfere with participant safety, as defined by medical history, physical examination (including vital signs), electrocardiogram (ECG), and routine laboratory tests as determined by the Principal Investigator (PI)/investigator. 5. Participants will have a d
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
59
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Not provided
Studied LA-formulation(s)
Not provided
Studied route(s) of administration
Not provided
Use case
Not provided
Key resources
CD388.IM.SQ.1.01
Identifier
NCT05285137
Link
https://clinicaltrials.gov/study/NCT05285137
Phase
Phase I
Status
Completed
Sponsor
Cidara Therapeutics Inc.
More details
The purpose of this first-in-human study is to determine the safety and tolerability profile of CD388 Injection, as compared to saline placebo, when administered as a single dose to healthy adult subjects by injection either in the muscle or under the skin.
Purpose
Study of CD388 Intramuscular or Subcutaneous Administration in Healthy Subjects
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-03-14
Anticipated Date of Last Follow-up
2025-02-12
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2023-10-27
Actual Completion Date
2023-10-27
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: 1. Willing and able to provide written informed consent. 2. Males and females 18 to 65 years of age, inclusive. 3. A female subject must meet one of the following criteria: 1. If of childbearing potential - agrees to use a highly effective, preferably user-independent method of contraception (failure rate of \<1 percent per year when used consistently and correctly) for at least 30 days prior to screening and agrees to remain on a highly effective method until 205 days after last dose of study medication. Examples of highly-effective methods of contraception include: abstinence from heterosexual intercourse; hormonal contraceptives (birth control pills, injectable/implant/insertable hormonal birth control products, transdermal patch); intrauterine device (with or w
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
77
Allocation
Randomized
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Not provided
Studied LA-formulation(s)
Not provided
Studied route(s) of administration
Not provided
Use case
Not provided
Key resources
CD388.SQ.1.03
Identifier
NCT05619536
Link
https://clinicaltrials.gov/study/NCT05619536
Phase
Phase I
Status
Completed
Sponsor
Cidara Therapeutics Inc.
More details
The purpose of this study is to determine the safety and tolerability profile of CD388 Injection, as compared to saline placebo, when dosed by subcutaneous (SQ) administration as a single dose to healthy Japanese adult subjects.
Purpose
Study of CD388 Subcutaneous Administration in Healthy Japanese Subjects
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-10-18
Anticipated Date of Last Follow-up
2024-06-24
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2023-07-14
Actual Completion Date
2023-07-14
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: 1. Must be of Japanese descent with Japanese parents and grandparents, as determined by subject's verbal report. 2. Willing and able to provide written informed consent. 3. Males and females 18 to 65 years of age, inclusive. 4. A female subject must meet one of the following criteria: 1. If of childbearing potential - agrees to use a highly effective, preferably user-independent method of contraception (failure rate of \<1 percent per year when used consistently and correctly) for at least 30 days prior to screening and agrees to remain on a highly effective method until 7 months after last dose of study medication, whichever is longer. Examples of highly-effective methods of contraception include: abstinence from heterosexual intercourse; hormonal contraceptives (
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
28
Allocation
Randomized
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Not provided
Studied LA-formulation(s)
Not provided
Studied route(s) of administration
Not provided
Use case
Not provided
Key resources
NAVIGATE
Identifier
NCT06609460
Link
https://clinicaltrials.gov/study/NCT06609460
Phase
Phase II
Status
Not provided
Sponsor
Cidara Therapeutics Inc.
More details
The purpose of this study is to evaluate the effectiveness of CD388 in preventing symptomatic laboratory-confirmed influenza infections, as compared to placebo, and to select a dose of CD388 that is effective in preventing the same, when administered as a single dose via 3 subcutaneous (SQ) injections to adult participants in stable health, and to evaluate the safety and tolerability of CD388, as compared to placebo.
Purpose
Study of CD388 for the Prevention of Influenza in Subjects Not at Risk for Influenza Complications
Interventions
Not provided
Countries
Not provided
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2024-09-20
Anticipated Date of Last Follow-up
2025-03-12
Estimated Primary Completion Date
2025-09-01
Estimated Completion Date
2025-09-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: 1. Willing and able to provide written informed consent and comply with scheduled visits, laboratory tests, and other study procedures. 2. Males and females 18 to less than 64 years of age. 3. In the Investigator's clinical judgment, is in stable health at the time of screening and randomization. Participants may not have underlying hematologic, oncologic, renal, autoimmune, and/or cardiopulmonary illnesses or be considered at risk of developing complications from influenza infection per the CDC guidelines (chronic obstructive pulmonary disease \[COPD\], asthma, immune compromised current cancer \[except non-melanomatous skin cancer\], or diabetes). Subjects will be included on the basis of medical history and vital signs taken between signing of the informed consent a
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
5000
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Not provided
Studied LA-formulation(s)
Not provided
Studied route(s) of administration
Not provided
Use case
Not provided
Key resources
Excipients
Proprietary excipients used
No proprietary excipient used
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
No novel excipient or existing excipient used
Residual solvents used
No residual solvent used
Additional features
Other features of the technology
- Drug-eluting
- Other(s)
Targeted action
Release properties
Small molecule conjugates in DFC exhibit selective targeting towards enzyme active sites and receptors. The distribution of the active pharmaceutical ingredient (API) is influenced by the molecular size of the DFC, with smaller sizes facilitating faster tissue penetration. The API is released from the Fc domain at the target site in a cumulative manner, optimizing therapeutic efficacy through sustained and controlled delivery for a longer period of time.
Injectability
Preclinical and clinical studies focused on DFC drug candidates administered via intramuscular (IM) and subcutaneous (SQ) routes. For administration, a 25-gauge or larger bore needle is used.
Safety
Interim analysis of Phase 2a studies of CD-388 shows that the drug was well-tolerated with no treatment emergent adverse events (TEAE) or serious adverse events (SAE) in 28 subjects who received CD-388.
Stability
Not provided
Storage conditions and cold-chain related features
The formulation should be stored at 20°C–25°C. It can also be stored at 5°C–25°C for up to 24 hours.
Potential application(s)
Therapeutic area(s)
Use case(s)
Use of technology
Ease of administration
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
Frequency of administration
Weekly, Single dose administration
User acceptance
Not provided
Targeted user groups
Age Cohort- Adults
- Older Adults
- All
Pregnant individuals
No
Lactating individuals
No
Healthy individuals
Unspecified
Comment
Not provided
Potential associated API(s)
Temsavir
Class(es)
Antiretroviral
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
HIV
Foreseen user group
Not provided
Foreseen duration between application(s)
Not provided
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
zanamivir
Class(es)
antiviral (neuraminidase inhibitor)
Development stage
Phase II
Clinical trial number(s)
NCT06609460
Foreseen/approved indication(s)
Seasonal influenza
Foreseen user group
Not provided
Foreseen duration between application(s)
Not provided
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Patent info
Description
Compositions and methods for the treatment of viral infections include conjugates containing inhibitors of viral neuraminidase and MoT influenza or parainfluenza
Brief description
Compositions and methods for the treatment of viral infections include conjugates containing inhibitors of viral neuraminidase (e.g., zanamivir, peramivir, or analogs thereof) linked to an Fc monomer, an Fc domain, and Fc-binding peptide, an albumin protein, or albumin-binding peptide. In particular, conjugates can be used in the treatment of viral infections (e.g., influenza viral infections).
Representative patent
WO2021046549
Category
Compound, Composition
Patent holder
Cidara Therapeutics, Inc
Exclusivity
Not provided
Expiration date
September 8, 2040
Status
Granted: ZA, US, CO, AU, JP, NZ, CN Pending: HK, AE, BR, CA, CL, CR, EA, EC, EP (LMICs: AL, MK, TR, BA, ME, KH, MA, MD, TN, RS), ID, IL, KR, MX, MY, PE, PH, SA, SG, TH, TW, UA, VN, IN Withdrawn: ARIPO
Description
Conjugates of inhibitors of viral gp120 receptor (e.g., temsavir or analogs thereof) linked to an Fc monomer, an Fc domain, and Fc-binding peptide and use for treating HIV
Brief description
Conjugates containing viral gp120 receptor inhibitors (e.g., temsavir, BMS-818251, DMJ-ll-121, BNM-IV-147, or analogues thereof) linked to an Fc monomer, an Fc domain, an Fc-binding peptide, an albumin protein, or an albumin-binding peptide are used in the treatment of viral infections. These conjugates are particularly useful in the treatment of HIV infections.
Representative patent
WO2020252393
Category
Compound
Patent holder
Cidara Therapeutics, Inc
Exclusivity
Not provided
Expiration date
June 12, 2040
Status
Pending: AU, CA, CN, JP, US Not in force: EP
Description
Variant Fc domain monomer with linked to cyclic heptapeptides and method for treatment of bacterial infection
Brief description
Conjugates with an Fc domain covalently bonded to one or more monomers or dimers of cyclic heptapeptides are among the compositions and methods used to treat bacterial infections. These small-molecule conjugates have the potential to treat gram-negative bacterial infections.
Representative patent
WO2018128826
Category
Compound
Patent holder
Cidara Therapeutics, Inc
Exclusivity
Not provided
Expiration date
December 20, 2037
Status
Filed and pending in US only
Supporting material
Publications
There are no publication
Additional documents
Useful links
There are no additional links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Illustrations

Drug Fragment Conjugate
Corporate presentation. (n.d.). https://www.cidara.com/wp-content/uploads/2023/09/Cidara-Corporate-Deck-September-2023.pdf