
|
Developed by 

|
Supported by 

|
Enhanced Antibody Drug Conjugates (ADC)
Developer(s)

|
Ambrx (acquired by Johnson & Johnson, Inc.) Originator
https://ambrx.com/
United States of America Ambrx, founded in 2003 in San Diego, California, emerged from pioneering work in synthetic biology, specifically with the goal of expanding the genetic code to enable precise modifications of proteins. This idea was initiated by co-founders Peter Schultz and others from The Scripps Research Institute. |
Sponsor(s)

|
Johnson & Johnson, Inc. https://www.jnj.com/ |
Partnerships

|
Zhejiang Medicine Co., Ltd https://www.zmc.top/en/ |

|
Novocodex Biopharmaceuticals Co., Ltd http://www.novocodex.cn/ |

|
Sino Biopharmaceutical., Ltd https://www.sinobiopharm.com/en/ |

|
Beigene https://www.beigene.com |
Technology information
Type of technology
Antibody conjugated drug molecule
Administration route
Intravenous, Oral, Subcutaneous, Topical (Rectal), Transdermal
Development state and regulatory approval
ARX788
Phase III
FDA granted orphan drug designation to ARX788 for the treatment of HER2-positive gastric cancer
Description
Antibody-drug conjugates (ADCs) employ site-specific antibodies incorporating non-natural amino acids, novel linker chemistries designed for both in vitro and in vivo stability, and a combination of existing and novel targeted receptor agonists as payloads. The primary mechanism of action for these ADCs involves the accumulation of free payload in the targeted tissues, which emerges as the predominant driver of biological activity. Due to this mechanism, the drug conjugate remains in the body for an extended period with increased plasma concentration peak stability compared to the parent drug.
Technology highlight
1) ADC contains a novel linker molecule that conjugates the drug molecule/ therapeutic protein with the human antibody. 2) Linker molecule is a synthetic amino acid (SAA) with site-specificity, homogenous, and undergoes stable conjugation. 3) These ADCs are based on AMBRX's proprietary genetic code technology i.e., noncanonical amino acids (ncAA) based protein engineering (amber codon (UAG) suppression) 4) These ADCs target human antigen-presenting immune cells, binding to them to subsequently activate specific cell signaling pathways. This leads to stimulation or inhibition of the targeted protein synthesis. Consequently leading to a sustained pharmacological action of the API payload at the target site.
Technology main components
1) Payload (API) (Drug that has a functional group that forms a covalent linkage with phosphate linker) 2) Targeting Ligand (eg: Chimeric, humanized, or human antibodies) 3) Linker Arm (Eg: Pyrophosphate ester; triphosphate ester; Tetraphosphate ester) 4) Phosphate Group All these ingredients combine to form a complex. This complex is stable extracellularly and labile intracellularly.
Information on the raw materials sourcing, availability and anticipated price
Not provided
Delivery device(s)
No delivery device
APIs compatibility profile
API desired features
Water-soluble molecules
Water-insoluble molecules
Small molecules
ADC has targeted enzyme inhibitors such as dihydrofolate reductase inhibitors and thymidylate synthase inhibitors, DNA intercalators, glucocorticoid receptor agonists, nuclear receptor agonists, anti-inflammatory agents, DNA cleavers, topoisomerase inhibitors, anthracycline family of drugs, vinca drugs, the mitomycins, bleomycin, the cytotoxic nucleosides, pteridine, diynenes, podophyllotoxins, differentiation inducers, and taxols. Cytotoxic drugs such as duocarmycins and CC-1065 and analogs of CBI, MCBI, doxorubicin, aolastatins, combretastatin, etc. Other drugs are glucocorticoid agonists.
Proteins
Biotherapeutics such as Monoclonal Antibodies (Eg: Brentuximab vedotin, Trastuzumab emtansine, Polatuzumab vedotin, Inotuzumab ozogamicin, Moxetumomab pasudotox) are targeted for the ADC formulation. Therapeutic proteins are also targeted as a payload for the ADC formulation.
Additional solubility data
Not provided
Additional stability data
Not provided
API loading: Maximum drug quantity to be loaded
75-90 wt%
API co-administration
2 different APIs : Not provided
LogP
Min: 3 Max: 6
Scale-up and manufacturing prospects
Scale-up prospects
Ambrx has a manufacturing capacity of 2000 liters for ADC formulations.
Tentative equipment list for manufacturing
Not provided
Manufacturing
1. Expression and purification of antibodies with non-natural amino acids 2. Synthesis of ADC payloads and linkers 3. Site-specific conjugation 4. Intact Mass determination 5. DAR determination and antibody preparation evaluation
Specific analytical instrument required for characterization of formulation
1. Mass Spectrometry (using Thermo Deconvolution 2.0 Software) 2. HPLC 3. Flow Cytometry 4. LCMS
Clinical trials
ARX788-1711
Identifier
NCT03255070
Link
https://clinicaltrials.gov/study/NCT03255070
Phase
Phase I
Status
Completed
Sponsor
Ambrx, Inc.
More details
This 2-part, Phase 1, open-label study will determine the recommended Phase 2 dose (RP2D) of ARX788 in subjects with advanced HER2 positive cancers and will assess the safety and anticancer activity in breast, gastric and other advanced HER2 positive solid tumors.
Purpose
A Dose-escalation, Expansion Study of ARX788, in Advanced Solid Tumors Subjects With HER2 Expression (ACE-Pan Tumor 01)
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2018-03-20
Anticipated Date of Last Follow-up
2024-01-31
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2023-09-13
Actual Completion Date
2023-10-18
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria * Age \>18 years * Life expectancy \>3 months. * Female or male subjects whose advanced HER2 expressing cancer has failed standard of care treatments, or for whom such therapy is not acceptable to the subject. Subjects with advanced breast, gastric cancer, or other solid tumor who test positive for HER2 by ASCO/CAP criteria (either IHC or FISH) must have received prior treatment with a trastuzumab containing therapy. Subjects who have been previously treated with pertuzumab, TDM-1, lapatinib, or other available and accessible HER2-directed therapies or investigational therapies are eligible. * Disease measurability: * Phase 1a: measurable or non-measurable disease per RECIST v 1.1. * Phase 1b
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
106
Allocation
Not provided
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
ARX517
Identifier
NCT04662580
Link
https://clinicaltrials.gov/study/NCT04662580
Phase
Phase I
Status
Recruiting
Sponsor
Ambrx, Inc.
More details
This is a phase 1 study to assess the safety and tolerability of ARX517 in adult subjects with Metastatic Castration-Resistant Prostate Cancer (mCRPC).
Purpose
ARX517 in Subjects With Metastatic Castration-Resistant Prostate Cancer
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-07-27
Anticipated Date of Last Follow-up
2024-05-08
Estimated Primary Completion Date
2025-12-01
Estimated Completion Date
2027-03-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Male
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Key Inclusion Criteria: * Male subjects ≥ 18 years at the first time of providing written informed consent. * Histologically confirmed prostate adenocarcinoma. * Documented metastatic disease and evidence of disease progression * Castration-resistant prostate cancer defined as surgical or medical castration with serum testosterone levels of ≤ 50 ng/dL (1.73 nM) at Screening. For patients who have not undergone an orchiectomy, must be undergoing treatment with a luteinizing hormone-releasing hormone (LHRH) agonist or antagonist and must agree to continue such therapy while on study treatment. * Prior receipt of the following for metastatic prostate cancer: * at least two lines of treatment * at least two Food and Drug Administration (FDA)-approved therapies
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
262
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
ACE-Breast-03
Identifier
NCT04829604
Link
https://clinicaltrials.gov/study/NCT04829604
Phase
Phase II
Status
Recruiting
Sponsor
Ambrx, Inc.
More details
A Global, Phase 2 Study of ARX788 in HER2-positive Metastatic Breast Cancer Patients who were previously treated with T-DXd
Purpose
ARX788 in HER2-positive, Metastatic Breast Cancer Subjects (ACE-Breast-03)
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-10-26
Anticipated Date of Last Follow-up
2024-06-10
Estimated Primary Completion Date
2025-06-01
Estimated Completion Date
2026-12-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Key Inclusion Criteria: * Age ≥ 18 years and older * Life expectancy ≥ 6 months * Unresectable or metastatic breast cancer subjects * Presence of at least one measurable lesion per RECIST v 1.1 * Subjects must have an adequate tumor sample available for confirmation of HER2 status * Subjects must have had prior treatment with no more than 5 prior regimens of systemic treatment in the metastatic setting. One of these prior treatments must have been treatment with T-DXd. * Subjects with stable brain metastases * Acute toxicities from any prior therapy, surgery, or radiotherapy must have resolved to Grade ≤1 as per the NCI-CTCAE v 5.0, except alopecia * Adequate organ functions * Willing and able to understand and sign an informed consent inform and to comply with all aspects of the protocol
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
71
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
ACE-Pan tumor-02
Identifier
NCT05041972
Link
https://clinicaltrials.gov/study/NCT05041972
Phase
Phase II
Status
Withdrawn
Sponsor
Ambrx, Inc.
More details
A Global Phase 2 Study to Evaluate the Efficacy and Safety of ARX788 for Selected HER2-mutated or HER2-amplified/overexpressed Solid Tumors (ACE-Pan tumor-02)
Purpose
ARX788 in Selected HER2-mutated or HER2-amplified/Overexpressed Solid Tumors (ACE-Pan Tumor-02)
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-11-05
Anticipated Date of Last Follow-up
2022-09-09
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-04-20
Actual Completion Date
2022-04-20
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Age ≥ 18 years and older * Life expectancy \> 3 months * Eastern Cooperative Oncology Performance Status ≤ 1 * HER2 status must be determined from a local Clinical Laboratory Improvement Amendments (CLIA) or equivalent-certified laboratory. * Cohort 1, Cohort 2, and Explanatory Cohort A: HER2 mutated subjects with pre-specified HER2 activating mutation. Subjects with HER2 mutations in NSCLC (Cohort 1), breast cancer (Cohort 2), and other solid tumors (Cohort A) who have not received prior HER2 antibody drug conjugate (ADC) treatment are eligible. * Cohort 3: Subjects with HER2 amplifications in biliary tract cancers (BTC) who have not received prior HER2 ADC treatment are eligible. * Cohort 4: Subjects with HER2 amplifications in colorectal cancer (CRC), ovarian.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
Not provided
Allocation
Not provided
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
PRO-ARX201-701
Identifier
NCT00778518
Link
https://clinicaltrials.gov/study/NCT00778518
Phase
Phase II
Status
Completed
Sponsor
Ambrx, Inc.
More details
Study to find the optimal dose of Growth Hormone Replacement in young adult patients suffering from childhood onset growth hormone deficiency (GHD).
Purpose
Safety, Tolerability and PK/PD Study in Young Adult Patients With Childhood Onset Growth Hormone Deficiency (GHD).
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2008-07-01
Anticipated Date of Last Follow-up
2009-10-09
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2009-10-01
Actual Completion Date
2009-10-01
Studied populations
Age Cohort
- Adults
Genders
- Male
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * 18-30 years old * GHD of childhood onset * completed growth * IGF-1 \<=2SDS * rhGH treatment naive * hGH levels below cut-off Exclusion Criteria: * History of malignancy or intracranial tumors * ECG abnormality * ICH * hepatic dysfunction * renal impairment * major medical conditions * inadequate T4 * positive for HBV, HCV, or HIV * alcohol or drug abuse
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
36
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Single blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
237527
Identifier
NCT06224673
Link
https://clinicaltrials.gov/study/NCT06224673
Phase
Phase II
Status
Not yet recruiting
Sponsor
Laura Huppert, MD, BA
More details
This phase II trial tests how well ARX788 works in treating patients diagnosed with HER2-low, locally advanced unresectable or metastatic breast cancer. ARX788 is an antibody-drug conjugate (ADC) that is given by infusion (diluted and injected slowly into veins). Antibodies are proteins which are naturally produced by the body's immune system to help fight infections. ARX788 consists of antibodies that have been attached to a toxin that has the potential to kill cancer cells. ARX788 sticks to a protein called human epidermal growth factor receptor (HER2), which is found on some breast cancer cells. Giving ARX788 may be safe and effective in treating patients with HER2-low locally advanced unresectable metastatic breast cancer.
Purpose
ARX788 for Treating Patients With HER2-low Locally Advanced Unresectable or Metastatic Breast Cancer
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2024-10-15
Actual Start Date
Not provided
Anticipated Date of Last Follow-up
2024-09-10
Estimated Primary Completion Date
2028-02-29
Estimated Completion Date
2028-02-29
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female participants age 18 years or greater with the ability to provide written informed consent for the study. * Eastern Cooperative Oncology Group (ECOG) score of 0-2. * Estimated life expectancy of at least at 6 months per investigator assessment. * Ability to understand and the willingness to sign a written informed consent document. * Pathologically documented HER2-low locally advanced unresectable or metastatic breast cancer. NOTE: human epidermal growth factor receptor 2 (HER2) low status determined by HER2 immunohistochemistry (IHC) 1+ or 2+ and no evidence of HER2 gene amplification by in situ hybridization (ISH)/fluorescence in situ hybridization (FISH), which can be documented from any tumor sample during the patient's cancer treatment history (earl
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
36
Allocation
Not provided
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
ZMC-ARX788-101
Identifier
NCT02512237
Link
https://clinicaltrials.gov/study/NCT02512237
Phase
Phase I
Status
Terminated
Sponsor
Zhejiang Medicine Co., Ltd.
More details
This is a 2-part, Phase 1 FIH study with Phase 1a designed to determine the maximum tolerated dose (MTD)/recommended Phase 2 dose (RP2D) in subjects with metastatic cancers with a human epidermal growth factor receptor 2 (HER2) test result that is in situ hybridization (ISH) positive (+) or immunohistochemistry (IHC) 3+ or 2+, and Phase 1b designed to assess anticancer activity and safety in three expansion cohorts: two different advanced breast cancer expansion cohorts (namely, for tumors that test as HER2 ISH positive or IHC3+ and for tumors that test as HER2 ISH negative with IHC 2+), and one advanced gastric cancer expansion cohort (for tumors that test as HER2 ISH positive or IHC3+).
Purpose
A Dose-escalation Study of ARX788, IV Administered in Subjects With Advanced Cancers With HER2 Expression
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2016-03-01
Anticipated Date of Last Follow-up
2020-06-03
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2017-01-01
Actual Completion Date
2017-01-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Life expectancy \>12 weeks. 2. BMI is between 18 to 32 kg/m2 3. Subjects whose advanced cancer has failed treatment or whose cancer has progressed following available standard therapy or for whom such therapy is not acceptable to the subject. Subjects whose tumor tissue local laboratory results are HER2 ISH positive or IHC3+ must have been previously treated with a HER2 targeting therapy (e.g. trastuzumab, in the country or region where such therapies are available and part of standard of care), or have failed SOC therapy. Subjects who have been previously treated with a HER2 targeting therapy such as trastuzumab or ado-trastuzumab emtansine are eligible. 4. Disease measurability: Phase 1a: measureable or non-measureable disease; Phase 1b: disease must be measureabl
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
9
Allocation
Not provided
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
Excipients
Proprietary excipients used
No proprietary excipient used
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
No novel excipient or existing excipient used
Residual solvents used
No residual solvent used
Additional features
Other features of the technology
- Drug-eluting
Release properties
Preclinical studies of ARX788 show that the API released from the ADC increases with time.
Injectability
18-22 gauge needle is used for the ADC injection infusion
Safety
Phase 1 clinical trials of ARX788 revealed no dose-limiting toxicities or treatment-related serious adverse events (TEAE). 28/30 (93.3%) patients experienced at least one drug-related adverse event (AE) and 13.3% experienced grade 3 ARX788-related AEs. Preclinical studies in monkeys demonstrated a favorable safety profile for ARX788.
Stability
1. Structural stability - Preclinical studies show that ADCs have robust plasma stability coupled with rapid release of payload in a lysosomal environment. 2. Formulation stability - As a formulation, the stability data of ADC has not yet been disclosed.
Storage conditions and cold-chain related features
Not provided
Potential application(s)
Therapeutic area(s)
Use case(s)
Use of technology
Ease of administration
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
Frequency of administration
Weekly, Monthly
User acceptance
Not provided
Targeted user groups
Age Cohort- Adults
- Older Adults
- All
Pregnant individuals
Unspecified
Lactating individuals
Unspecified
Healthy individuals
No
Comment
Not provided
Potential associated API(s)
ARX788
Class(es)
anti-HER2 antibody drug conjugate
Development stage
Phase III
Clinical trial number(s)
NCT04829604
Foreseen/approved indication(s)
HER2-positive gastric cancer, advanced or metastatic HER2-positive breast cancer, and other solid tumors
Foreseen user group
Adults < 18 years of old having HER-2 positive gastric cancer
Foreseen duration between application(s)
Every 3 weeks
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
FDA granted orphan drug designation to ARX788 for the treatment of HER2-positive gastric cancer
ARX517
Class(es)
anti-PSMA antibody drug conjugate
Development stage
Phase I/II
Clinical trial number(s)
NCT04662580
Foreseen/approved indication(s)
Prostate cancer
Foreseen user group
Adults who are <18 years old with metastatic castration-resistant prostate cancer
Foreseen duration between application(s)
Every 3 weeks; Every 4 weeks
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
ARX305
Class(es)
anti-CD-70 antibody drug conjugate
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Not provided
Foreseen user group
Not provided
Foreseen duration between application(s)
Not provided
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
ARX107
Class(es)
IL2 cytokine (PEGlated)
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Not provided
Foreseen user group
Not provided
Foreseen duration between application(s)
Not provided
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Patent info
Description
Antibody drug conjugate for anti-inflammatory applications
Brief description
Antibody-drug conjugates (ADCs) comprising an antibody conjugated to an anti-inflammatory therapeutic agent via a phosphate-based linker with tunable extracellular and intracellular stability are described.
Representative patent
US11510993B2
Category
Not provided
Patent holder
Merck Sharp and Dohme LLC Ambrx Inc
Exclusivity
Not provided
Expiration date
November 15, 2038
Status
Active
Description
Phosphate based linkers for intracellular delivery of drug conjugates
Brief description
Phosphate-based linkers with tunable stability for intracellular delivery of drug conjugates are described. The phosphate-based linkers comprise a monophosphate, diphosphate, triphosphate, or tetraphosphate group (phosphate group) and a linker arm comprising a tuning element and optionally a spacer. A payload is covalently linked to the phosphate group at the distal end of the linker arm and the functional group at the proximal end of the linker arm is covalently linked to a cell-specific targeting ligand such as an antibody. These phosphate-based linkers have differentiated and tunable stability in blood vs. an intracellular environment (e.g. lysosomal compartment).
Representative patent
US10550190B2
Category
Not provided
Patent holder
Ambrx Inc; Merck Sharp and Dohme LLC
Exclusivity
Not provided
Expiration date
March 12, 2036
Status
Active
Description
Prostate-specific membrane antigen antibody drug conjugates
Brief description
This invention relates to prostate-specific membrane antigen (PSMA) antibodies and antibody drug conjugates comprising at least one non-naturally-encoded amino acid. Disclosed herein are αPSMA antibodies with one or more non-naturally encoded amino acids and further disclosed are antibody drug conjugates wherein the αPSMA antibodies of the invention are conjugated to one or more toxins. Also disclosed herein are non-natural amino acid dolastatin analogs that are further modified post-translationally, methods for effecting such modifications, and methods for purifying such dolastatin analogs. Typically, the modified dolastatin analogs include at least one oxime, carbonyl, dicarbonyl, and/or hydroxylamine group. Further disclosed are methods for using such non-natural amino acid antibody dru
Representative patent
US20220033518A1
Category
Formulation
Patent holder
Ambrx Inc
Exclusivity
Not provided
Expiration date
March 2, 2022
Status
Pending
Supporting material
Publications
<p><span style="color: rgb(27, 27, 27);">Lu, H., Wang, D., Kazane, S., Javahishvili, T., Tian, F., Song, F., Sellers, A., Barnett, B., & Schultz, P. G. (2013). Site-specific antibody-polymer conjugates for siRNA delivery. </span><em style="color: rgb(27, 27, 27);">Journal of the American Chemical Society</em><span style="color: rgb(27, 27, 27);">, </span><em style="color: rgb(27, 27, 27);">135</em><span style="color: rgb(27, 27, 27);">(37), 13885–13891. </span><a href="https://doi.org/10.1021/ja4059525" rel="noopener noreferrer" target="_blank" style="color: rgb(27, 27, 27);">https://doi.org/10.1021/ja4059525</a></p>
We describe here the development of site-specific antibody-polymer conjugates (APCs) for the selective delivery of small interference RNAs (siRNAs) to target cells. APCs were synthesized in good yields by conjugating an aminooxy-derivatized cationic block copolymer to an anti-HER2 Fab or full length IgG by means of genetically encoded para-acetyl phenylalanine (pAcF). The APCs all showed comparable binding affinity to HER2 as their native counterparts and no significant cellular cytotoxicity. Mutant S202-pAcF Fab and Q389-pAcF IgG polymer conjugates specifically delivered siRNAs to HER2+ cells and mediated potent gene silencing at both the mRNA and protein levels. However, a mutant A121-pAcF IgG polymer conjugate, despite its high binding affinity to HER2 antigen, did not induce a significant RNA interference response in HER2+ cells, presumably due to steric interference with antigen binding and internalization. These results highlight the importance of conjugation site on the activity of antibody-polymer based therapeutics and suggest that such chemically-defined APCs may afford a useful targeted delivery platform for siRNAs or other nucleic acid based therapies.
<p><span style="color: rgb(27, 27, 27);">Jackson, D., Atkinson, J., Guevara, C. I., Zhang, C., Kery, V., Moon, S. J., Virata, C., Yang, P., Lowe, C., Pinkstaff, J., Cho, H., Knudsen, N., Manibusan, A., Tian, F., Sun, Y., Lu, Y., Sellers, A., Jia, X. C., Joseph, I., Anand, B., … Stover, D. (2014). In vitro and in vivo evaluation of cysteine and site specific conjugated herceptin antibody-drug conjugates. </span><em style="color: rgb(27, 27, 27);">PloS one</em><span style="color: rgb(27, 27, 27);">, </span><em style="color: rgb(27, 27, 27);">9</em><span style="color: rgb(27, 27, 27);">(1), e83865. </span><a href="https://doi.org/10.1371/journal.pone.0083865" rel="noopener noreferrer" target="_blank" style="color: rgb(27, 27, 27);">https://doi.org/10.1371/journal.pone.0083865</a></p>
We report the results from the first direct preclinical comparison of a site specific non-natural amino acid anti-Her2 ADC and a cysteine conjugated anti-Her2 ADC. We report that the site specific non-natural amino acid anti-Her2 ADCs have superior in vitro serum stability and preclinical toxicology profile in rats as compared to the cysteine conjugated anti-Her2 ADCs. We also demonstrate that the site specific non-natural amino acid anti-Her2 ADCs maintain their in vitro potency and in vivo efficacy against Her2 expressing human tumor cell lines. Our data suggests that site specific non-natural amino acid ADCs may have a superior therapeutic window than cysteine conjugated ADCs.
<p>Skidmore L, Sakamuri S, Knudsen NA, et al. ARX788, a site-specific anti-HER2 antibody-drug conjugate, demonstrates potent and selective activity in HER2-low and T-DM1–resistant breast and gastric cancer. <em>Mol Cancer Ther</em>. 2020;19(9):1833-1843. doi:<a href="10.1158/1535-7163.MCT-19-1004" rel="noopener noreferrer" target="_blank">10.1158/1535-7163.MCT-19-1004</a></p><p><br></p>
First-generation antibody-drug conjugates (ADC) are heterogeneous mixtures that have shown clinical benefit, but generally exhibited safety issues and a narrow therapeutic window due, in part, to off-target toxicity caused by ADC instability. ARX788 is a next-generation, site-specific anti-HER2 ADC that utilizes a unique nonnatural amino acid-enabled conjugation technology and a noncleavable Amberstatin (AS269) drug-linker to generate a homogeneous ADC with a drug-to-antibody ratio of 1.9. ARX788 exhibits high serum stability in mice and a relatively long ADC half-life of 12.5 days. When compared in vitro against T-DM1 across a panel of cancer cell lines, ARX788 showed superior activity in the lower HER2-expressing cell lines and no activity in normal cardiomyocyte cells. Similarly, ARX788 significantly inhibited tumor growth, and generally outperformed T-DM1 in HER2-high and HER2-low expression xenograft models. Breast and gastric cancer patient-derived xenograft studies confirmed strong antitumor activity of ARX788 in HER2-positive and HER2-low expression tumors, as well as in a T-DM1-resistant model. The encouraging preclinical data support the further development of ARX788 for treatment of patients with HER2-positive breast and gastric cancer, including those who have developed T-DM1 resistance, and patients with HER2-low expression tumors who are currently ineligible to receive HER2-targeted therapy.
Additional documents
No documents were uploaded
Useful links
There are no additional links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Illustrations
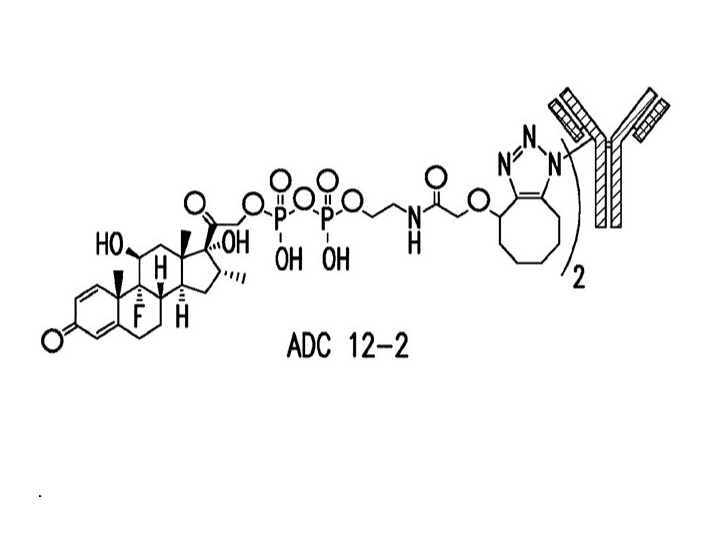
Antibody-drug conjugate (ADC)
Ambrx, Inc. (2020). Antibody-drug conjugates and methods of use (U.S. Patent No. 10,550,190). U.S. Patent and Trademark Office. https://patents.google.com/patent/US10550190B2/en

3D illlustration of ADC
Ambrx. (n.d.). Enhanced technology. Retrieved November 7, 2024, from https://ambrx.com/technology/#enhanced
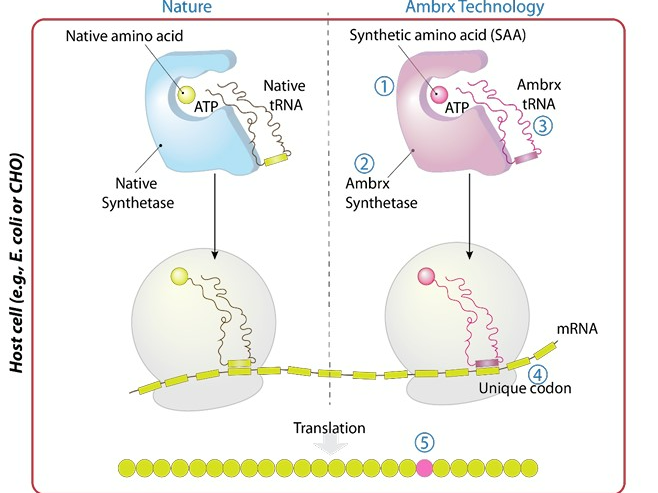
Intracellular Mechanism of Action of ADC Technology
Ambrx. (n.d.). Enhanced technology. Retrieved November 7, 2024, from https://ambrx.com/technology/#enhanced