
|
Developed by 

|
Supported by 


|
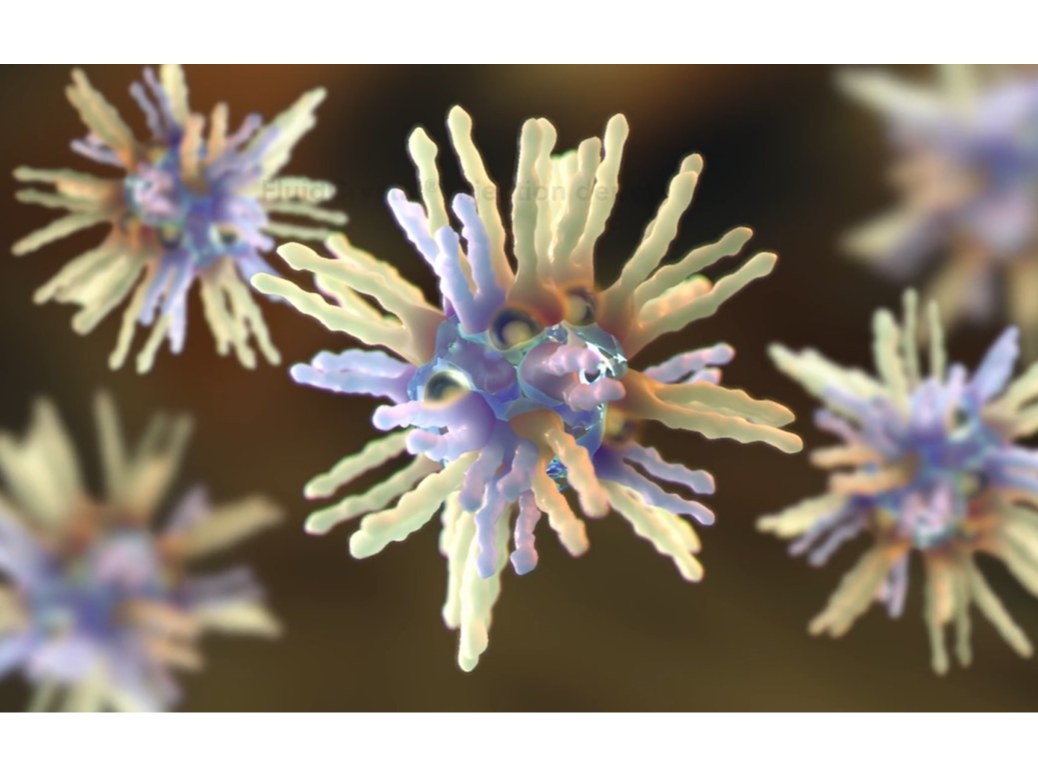
FluidCrystal®
Based on public informationDeveloper(s)

|
Camurus AB Originator
https://www.camurus.com
Sweden Camurus AB is a biopharmaceutical company that creates long-acting treatments for serious and chronic diseases. This company originated in 1991. Camurus, based on scientific understanding, aims to enhance patient outcomes through innovative therapies. They specialise in pharmaceutical commercialization and have a portfolio that includes clinical trial products as well as two FDA-approved drugs. |
Sponsor(s)
|
No sponsor indicated |
Partnerships

|
Braeburn Pharmaceuticals https://braeburnrx.com/ |

|
Rhythm Pharmaceuticals https://rhythmtx.com/ |

|
NewBridge Pharmaceuticals https://www.nbpharma.com/ |

|
Novartis https://www.novartis.com/ |
Technology information
Type of technology
Based on other organic particles, Amphiphile-based
Administration route
Subcutaneous, Intravenous, Optical
Development state and regulatory approval
Buprenorphine
Marketed
Buprenorphine long acting is approved under the brand name - Brixadi by US FDA and as Buvidal by EMA for Opoid Dependence.
Description
FluidCrystal Technology is an advanced injectable formulation utilizing endogenous lipids, characterized by low viscosity and long-acting properties. This technology enables the administration of active pharmaceutical ingredients (APIs) with controlled release durations ranging from days to months. The mechanism underlying this system involves reversed non-lamellar liquid crystalline phases, which facilitate the efficient encapsulation of APIs. These phases ensure a sustained and steady-state release of the API into the bloodstream, thereby enhancing therapeutic efficacy.
Technology highlight
• Self assembling lipids forms a nanostructure reversed-phase nonlamellar liquid crystal around the API in an aqueous environment • Easy and convenient administration • High treatment adherence • Adapted to prefilled syringes and pen injection devices • Small injection volume with a thin needle.
Technology main components
The level of each component varies based on the physiological and chemical properties of the API a) One or more neutral diacyl lipids and / or at least one tocopherol b) One or more 5–90% phospholipids c) One or more biocompatible , oxygen-containing, or low-viscosity organic solvents d) At least one bioactive agent is dissolved or dispersed in the low-viscosity mixture e) One or more oxygen-containing solvent selected from alcohols , ketones , esters , ethers , amides and sulfoxide
Information on the raw materials sourcing, availability and anticipated price
The price of Brixadi (FDA approved FluidCrystal drug) is 459.13 USD. Camurus obtains commercially available, high-quality sources of key components for FluidCrystal formulation.
Delivery device(s)
No delivery device
APIs compatibility profile
API desired features
Water-soluble molecules
Water-insoluble molecules
Small molecules
Small molecules that are targeted for FluidCrystal Technology are Opioids, local analgesics, hormones, anti-emetics, local antibiotics, and prostacyclins
Proteins
Peptides & proteins that are targeted for FluidCrystal Technology are somatostatin & analogues, LHRH agonists, Glucagon & insulin, GLP-1 & analogues, MC4 agonists, and antibody fragments
Additional solubility data
Not provided
Additional stability data
Not provided
API loading: Maximum drug quantity to be loaded
< 10 wt%
API co-administration
Not provided
LogP
Not provided
Scale-up and manufacturing prospects
Scale-up prospects
In 2022, 830,000 doses were manufactured
Tentative equipment list for manufacturing
Not provided
Manufacturing
Manufacturing and distribution of Camurus’ products is based on a multi-stage supply chain with several participants involved. Braeburn Pharmaceuticals overlooks the manufacturing of the FDA approved drugs of FluidCrystal in liaison with a third party manufacturer. The manufacturing process includes: - Compounding - Filtration - Filing The aim of the manufacturing process setup is to reduce product manufacturing waste by 20% and to increase the use of packaging material originating from sustainable sources to at least 50%.
Specific analytical instrument required for characterization of formulation
The listed analytical instrument used was HPLC with UV detection
Clinical trials
CAM2038
Identifier
NCT02946073
Link
https://clinicaltrials.gov/study/NCT02946073
Phase
Phase III
Status
Completed
Sponsor
Braeburn Pharmaceuticals
More details
This is a Phase III, placebo-controlled, multicenter study with an enriched-enrollment withdrawal (EEW) design to evaluate the efficacy and safety of CAM2038 in opioid-experienced subjects with moderate to severe CLBP that requires continuous, around-the-clock (ATC) opioid treatment ≥ 40 mg morphine equivalent dose (MED). The study includes 5 phases: A Screening Phase (up to 2 weeks), a Transition Phase (up to 2 weeks), an Open-Label Titration Phase (up to 10 weeks), a Double-Blind Treatment Phase including a Final Study Visit (12 weeks), and a Follow-up Phase (4 weeks). The overall duration of participation in the core phase of the study (randomized Double-Blind Phase) is up to 30 weeks, from the Screening Phase through the Follow-up Phase. Subjects who complete the Double-Blind Treatment
Purpose
Buprenorphine (CAM2038) in Subjects With a Recent History of Moderate to Severe Chronic Low Back Pain
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2016-09-01
Anticipated Date of Last Follow-up
2021-09-20
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2018-05-01
Actual Completion Date
2019-02-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Written informed consent provided prior to the conduct of any study-related procedures. 2. Male or non-pregnant, non-lactating female subject, greater than or equal to 18 years old. 3. Body mass index (BMI) between 18 and 38 kg/m2, inclusive. 4. Treated with daily opioids for moderate to severe CLBP for a minimum of 3 months prior to Screening. 5. On a stable dose of ≥40 mg/day of oral morphine or MED during the 14 days prior to Screening. 6. Systolic blood pressure ≥100 mmHg and diastolic blood pressure ≥60 mmHg. 7. Female subject of childbearing potential who is willing to use a reliable method of contraception during the entire study (Screening Visit to final Follow-up). To be considered not of childbearing potential, female subjects must be surgically sterile.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
1053
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
ACROINNOVA 1
Identifier
NCT04076462
Link
https://clinicaltrials.gov/study/NCT04076462
Phase
Phase III
Status
Completed
Sponsor
Camurus AB
More details
The purpose of this trial is to assess the efficacy and safety of CAM2029 in patients with acromegaly. Patients will be randomized to either CAM2029 or placebo administered subcutaneously once monthly during 6 months.
Purpose
A Trial to Assess Efficacy and Safety of Octreotide Subcutaneous Depot in Patients With Acromegaly
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-08-19
Anticipated Date of Last Follow-up
2024-04-24
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2023-05-02
Actual Completion Date
2023-05-02
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female patients, ≥18 years at screening * Able to provide written informed consent to participate in the trial prior to any trial related procedures are performed * Diagnosis of acromegaly by historical evidence of (persistent or recurrent) acromegaly * Treatment with a stable dose of octreotide LAR or lanreotide ATG for at least 3 months as monotherapy prior to screening * IGF-1 levels ≤1xULN at screening * Adequate liver, pancreatic, renal and bone marrow functions * Normal ECG Exclusion Criteria: * GH ≥2.5 μg/L at screening (cycle) * Have received medical treatment for acromegaly with pasireotide (within 6 months prior to screening), pegvisomant (within 3 months prior to screening), dopamine agonists (within 3 months prior to screening).
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
72
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
HS-12-455
Identifier
NCT02299089
Link
https://clinicaltrials.gov/study/NCT02299089
Phase
Phase II
Status
Completed
Sponsor
Camurus AB
More details
This is a Phase II, open-label multicentre, randomised study to assess the PK, PD, efficacy, and safety of two dosing regimens of CAM2029 in adult patients with acromegaly or a functional, well-differentiated NET, with carcinoid symptoms.
Purpose
Phase II Study of Subcutaneous Inj. Depot of Octreotide in Patients With Acromegaly and Neuroendocrine Tumours (NETs)
Interventions
Intervention 1
Countries
Not provided
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2015-01-01
Anticipated Date of Last Follow-up
2017-05-16
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2016-05-01
Actual Completion Date
2016-06-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: Acromegaly: * Male or female patients ≥18 years of age * Acromegaly currently treated with Sandostatin LAR NET: * Male or female patients ≥18 years of age * Functional, well-differentiated (Grade 1 or Grade 2) NET with symptoms of carcinoid syndrome (number of bowel movements and/or flushing) * Currently treated with Sandostatin LAR for symptom control Exclusion Criteria: Acromegaly: * Inadequate bone marrow function * Abnormal coagulation or chronic treatment with warfarin or coumarin derivates * Impaired liver, cardiac and/or renal function * Known gallbladder, bile duct disease or pancreatitis * Diabetes with poorly controlled blood glucose levels despite adequate therapy * Hypothyroidisms not adequately treated.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
12
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
SORENTO
Identifier
NCT05050942
Link
https://clinicaltrials.gov/study/NCT05050942
Phase
Phase III
Status
Not provided
Sponsor
Camurus AB
More details
The purpose of this study is to compare the effectiveness and safety of CAM2029 to octreotide LAR or lanreotide ATG in patients with advanced, well-differentiated GEP-NET. Patients who experience progressive disease in the randomized part of the study may proceed to an open-label extension part with intensified treatment with CAM2029.
Purpose
A Trial to Assess Efficacy and Safety of Octreotide Subcutaneous Depot in Patients With GEP-NET
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-10-22
Anticipated Date of Last Follow-up
2024-01-25
Estimated Primary Completion Date
2024-12-01
Estimated Completion Date
2026-12-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female patient ≥18 years old * Histologically confirmed, advanced (unresectable and/or metastatic), and well-differentiated NET of GEP or presumed GEP origin * At least 1 measurable, somatostatin receptor-positive lesion according to RECIST 1.1 determined by multiphasic CT or MRI (performed within 28 days before randomization) * ECOG performance status of 0 to 2 Exclusion Criteria: * Documented evidence of disease progression while on treatment (including SSAs) for locally advanced unresectable or metastatic disease * Known central nervous system metastases * Consecutive treatment with long-acting SSAs for more than 6 months before randomization * Carcinoid symptoms that are refractory to treatment (according to the Investigator's judgement) with convention.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
332
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
HS-19-647
Identifier
NCT04125836
Link
https://clinicaltrials.gov/study/NCT04125836
Phase
Phase III
Status
Not provided
Sponsor
Camurus AB
More details
The purpose of this trial is to assess the long-term safety and efficacy of CAM2029 in patients with acromegaly. Patients will be administered CAM2029 subcutaneously once monthly during 12 months. Patients fulfilling trial NCT04076462 will be offered to continue with open-label treatment week 24-52 in this trial. Patients completing the main part of the trial will be offered 52 weeks continued open-label treatment in an extension part.
Purpose
A Trial to Assess the Long-term Safety of Octreotide Subcutaneous Depot in Patients With Acromegaly
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2019-10-10
Anticipated Date of Last Follow-up
2024-04-10
Estimated Primary Completion Date
2025-06-01
Estimated Completion Date
2025-06-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female patients, ≥18 years at screening * Able to provide written informed consent to participate in the trial * Diagnosis of acromegaly by historical evidence of (persistent or recurrent) acromegaly * Treatment with a stable dose of octreotide LAR or lanreotide ATG for at least 3 months as monotherapy prior to screening * IGF-1 levels ˃1xULN and ≤2.0xULN at screening or IGF-1 levels ≤1xULN at screening with or without prior pituitary radiotherapy * Adequate liver, pancreatic, renal and bone marrow functions * Normal ECG Exclusion Criteria: For Roll-over Patients from NCT04076462: * Unresolved, drug-related serious adverse event (SAE) from the preceding trial.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
135
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
POSITANO
Identifier
NCT05281328
Link
https://clinicaltrials.gov/study/NCT05281328
Phase
Phase II/III
Status
Not provided
Sponsor
Camurus AB
More details
The purpose of the trial is to compare the effectiveness and safety of 2 treatment regimens of CAM2029 (given weekly or every 2 weeks) to placebo in participants with symptomatic PLD, either isolated as in autosomal dominant PLD (ADPLD) or associated with autosomal dominant polycystic kidney disease (ADPKD). In the Treatment Period of the trial, participants will be allocated at random to 1 of the 3 treatment arms in a 1:1:1 ratio. After completing the Treatment Period (53 weeks) participants may proceed to a 24-week open-label extension part of the trial and then only receive the same CAM2029 treatment. The active ingredient in CAM2029, octreotide, is administered as a subcutaneous depot using Camurus' FluidCrystal® technology.
Purpose
A Trial to Assess the Efficacy and Safety of Octreotide Subcutaneous Depot in Patients With PLD
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-06-28
Anticipated Date of Last Follow-up
2024-02-13
Estimated Primary Completion Date
2025-02-01
Estimated Completion Date
2025-08-01
Actual Primary Completion Date
2011-05-01
Actual Completion Date
2011-06-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Male or female patient, ≥18 years at screening * Diagnosis of PLD (associated with ADPKD or isolated as in ADPLD) as defined by htTLV ≥1800 mL/m at screening * Presence of at least 1 of the following PLD-related symptoms within 2 weeks before screening: bloating, fullness in abdomen, lack of appetite, feeling full quickly after beginning to eat, acid reflux, nausea, rib cage pain or pressure, pain in side, abdominal pain, back pain, shortness of breath after physical exertion, limited in mobility, concern about abdomen getting larger, dissatisfied by the size of abdomen * Not a candidate for, or not willing to undergo, surgical intervention for hepatic cysts during the trial.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
71
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Quadruple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
HS-12-460
Identifier
NCT02212197
Link
https://clinicaltrials.gov/study/NCT02212197
Phase
Phase II
Status
Completed
Sponsor
Camurus AB
More details
The purpose of this study is to assess the pharmacokinetics, pharmacodynamics, efficacy and safety of CAM2032 versus Eligard, in patients with prostate cancer. All patients will receive leuprolide acetate administered subcutaneously once monthly during 3 months.
Purpose
Phase II Study of Subcutaneous Injection Depot of Leuprolide Acetate in Patient With Prostate Cancer
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2014-09-01
Anticipated Date of Last Follow-up
2017-03-15
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2015-11-01
Actual Completion Date
2016-03-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Male
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Men ≥40 and ≤85 years of age * Histological or cytological proven adenocarcinoma of the prostate requiring hormone therapy * Life expectancy over 12 months * World Health Organisation/ The Eastern Cooperative Oncology Group (WHO/ECOG) performance status of 0, 1 or 2 * Adequate and stable renal function * Adequate and stable hepatic function Exclusion Criteria: * Evidence of brain metastasis, spinal cord compression, or urinary tract obstruction * Serum Testosterone levels below 150 ng/dL at Screening visit * Medical or radiological prostate cancer treatments within 2 months prior to the Screening visit * Surgical treatment of prostate cancer within 2 weeks prior to the Screening visit * Prior orchiectomy, hypophysectomy, or adrenalectomy * Prior use of LHRH agonist
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
51
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
Excipients
Proprietary excipients used
No proprietary excipient used
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
No novel excipient or existing excipient used
Residual solvents used
No residual solvent used
Additional features
Other features of the technology
- Biodegradable
- Drug-eluting
- Monolithic
Release properties
Upon administration of the FluidCrystal SC injection, lamellar liquid crystal nanoparticles are formed by phospholipids in conjunction with the active pharmaceutical ingredient (API). This structural formation results in an initial burst release of the API into the fatty tissue environment. Subsequently, the plasma concentration of the API gradually decreases, yielding a near dose-proportional drug release over a target period of four weeks. Furthermore, repeated weekly administrations of the FluidCrystal product demonstrate smaller and less frequent fluctuations in the plasma concentration.
Injectability
Use a 23 to 25 gauge, 5/8-inch needle for the subcutaneous FluidCrystal injection. Clean the injection site and inject into the subcutaneous tissue of the abdomen, upper arm, or thigh, rotating sites each time.
Safety
A recent study aimed to evaluate the safety and efficacy of Buprenorphine administered via weekly or monthly subcutaneous (SC) injection using the FluidCrystal technology, compared to the traditional daily sublingual administration. Among the 215 patients assigned to receive SC Buprenorphine, 128 reported experiencing at least one adverse effect. The most frequently observed adverse events included injection-site pain, headache, constipation, nausea, as well as injection-site pruritus and erythema.
Stability
Not provided
Storage conditions and cold-chain related features
FluidCrystal formulations are should be stored at room temperature, specifically between 20ºC to 25ºC . Within the range of 15°C to 30°C, fluctuations are allowed. In case of unexpected temperature variations, products should have a fall-back at refrigerated storage conditions.
Potential application(s)
Therapeutic area(s)
Use case(s)
Use of technology
Ease of administration
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
- Self-administered
Frequency of administration
Weekly, Monthly
User acceptance
Not provided
Targeted user groups
Age Cohort- Adults
- Older Adults
- All
Pregnant individuals
Unspecified
Lactating individuals
Unspecified
Healthy individuals
Unspecified
Comment
Not provided
Potential associated API(s)
Buprenorphine
Class(es)
Analgesics
Development stage
Marketed
Clinical trial number(s)
NCT02651584
Foreseen/approved indication(s)
Opoid Dependence and Chronic Pain Management
Foreseen user group
All genders above the age of 18 years
Foreseen duration between application(s)
Once weekly (8mg, 16mg, 24mg and 32mg) and Once monthly injections (64mg, 96mg and 128mg)
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Buprenorphine long acting is approved under the brand name - Brixadi by US FDA and as Buvidal by EMA for Opoid Dependence.
Pituitary and hypothalamic hormones and analogues
Class(es)
Synthetic Stomatostatin
Development stage
Phase III
Clinical trial number(s)
NCT04076462
Foreseen/approved indication(s)
Acromegaly, Gastroenteropancreatic neuroendocrine tumors and Polycystic liver disease
Foreseen user group
Not provided
Foreseen duration between application(s)
Once monthly
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
holds Orphan Drug Designation in the EU for CAM2029 for the treatment of acromegaly
Patent info
Description
Lipid Depot Formulations
Brief description
The invention relates to pre-formulations made from low viscosity, non-liquid crystalline mixtures of a neutral diacyl lipid, tocopherol, phospholipid, and a biocompatible organic solvent. The bioactive agent is dissolved in the mixture, and the pre-formulation forms a liquid crystalline phase structure upon contact with an aqueous fluid. These preformulations are suitable for generating depot compositions for sustained release of active agents. The invention also relates to methods of delivery, treatment, and the use of preformulations in the manufacture of a medicament.
Representative patent
WO2005117830
Category
formulation
Patent holder
Camurus AB
Exclusivity
Not provided
Expiration date
June 6, 2035
Status
Granted in : AU, BR, CA, CN, IL, JP, KR, MX, NZ, RU, ZA, UA, EP (GB, DK, FR, DE, IS, IE, IT, NL, PL, ES, SE, CH, TR,) SG, US
Description
Depot precursor formulation comprising buprenorphine for sustained delivery of and for treatment of pain or opioid dependence
Brief description
A depot precursor formulation comprising: a) a controlled-release matrix; b) at least oxygen containing organic solvent; c) at least 12% by weigh of at least one active agent selected from buprenorphine and salts thereof, calculated as buprenorphine free base. Corresponding depot compositions and methods of treatment in pain management, by opioid maintenance and related methods are provided.
Representative patent
WO2014016428
Category
formulation
Patent holder
Camurus AB
Exclusivity
Not provided
Expiration date
July 26, 2033
Status
Granted in: AU, BR, CA, CL, CN, CO, IN, ID, JP, KR, MX, NZ, PE, SG, ZA, TH, US, EA (AM, AZ ,BY, KZ, RU, TJ, TM), EP (AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LI, LT, LU, LV, MC, NL, NO, PL, PT, RO, RS, SE, SI, SK, TR) Pending in: AR, IL, MY, HK
Description
Composition for the delayed delivery of a peptide active agent
Brief description
A composition for the delayed delivery of a peptide active agent comprising; i) a salt of said peptide active agent comprising at least one positively charged peptide ion and at least one negatively charged counter-ion ii) a sustained-release delivery vehicle. Wherein said at least one negatively charged counter-ion is a halide ion, preferably a chloride or bromide ion.
Representative patent
WO2008152401
Category
formulation
Patent holder
Camurus AB
Exclusivity
Not provided
Expiration date
June 13, 2028
Status
Not provided
Supporting material
Publications
<p><span style="color: rgb(33, 33, 33);">Albayaty, M., Linden, M., Olsson, H., Johnsson, M., Strandgården, K., & Tiberg, F. (2017). Pharmacokinetic Evaluation of Once-Weekly and Once-Monthly Buprenorphine Subcutaneous Injection Depots (CAM2038) Versus Intravenous and Sublingual Buprenorphine in Healthy Volunteers Under Naltrexone Blockade: An Open-Label Phase 1 Study. </span><em style="color: rgb(33, 33, 33);">Advances in therapy</em><span style="color: rgb(33, 33, 33);">, </span><em style="color: rgb(33, 33, 33);">34</em><span style="color: rgb(33, 33, 33);">(2), 560–575.</span><a href=" https://doi.org/10.1007/s12325-016-0472-9" rel="noopener noreferrer" target="_blank" style="color: rgb(33, 33, 33);"> https://doi.org/10.1007/s12325-016-0472-9</a></p>
A study involving 89 healthy volunteers was conducted to evaluate the effectiveness of a new treatment for buprenorphine. The participants were divided into five groups: intravenous buprenorphine 600 µg, sublingual buprenorphine 8, 16, or 24 mg daily for 7 days, or four repeated weekly doses of CAM2038 q1w 16 mg. All subjects received daily naltrexone. The mean duration of buprenorphine release after CAM2038 q4w was 4-10 hours, with a mean terminal half-life of 19-25 days. Both CAM2038 formulations showed complete absolute bioavailability of buprenorphine, with 5.7- to 7.7-fold greater bioavailability compared to sublingual buprenorphine. Both CAM2038 q1w and q4w were well tolerated, with higher acceptance rates for CAM2038 than sublingual buprenorphine 1 hour post-dose.
<p><span style="color: rgb(34, 34, 34);">Chang, D. P., Barauskas, J., Dabkowska, A. P., Wadsäter, M., Tiberg, F., & Nylander, T. (2015). Non-lamellar lipid liquid crystalline structures at interfaces. </span><em style="color: rgb(34, 34, 34);">Advances in colloid and interface science</em><span style="color: rgb(34, 34, 34);">, </span><em style="color: rgb(34, 34, 34);">222</em><span style="color: rgb(34, 34, 34);">, 135-147.</span><a href=" https://doi.org/10.1016/j.cis.2014.11.003" rel="noopener noreferrer" target="_blank" style="color: rgb(34, 34, 34);"> </a><a href="https://doi.org/10.1016/j.cis.2014.11.003" rel="noopener noreferrer" target="_blank" style="color: rgb(31, 31, 31);">https://doi.org/10.1016/j.cis.2014.11.003</a></p>
Non-lamellar interfacial layer: from the organization of the adsorbed layer to the characterization of the internal structure. The self-assembly of lipids results in the formation of various nano-structures, including non-lamellar liquid crystalline structures like cubic, hexagonal, and sponge phases. These non-lamellar phases are crucial for living systems, providing compartmentalization and acting as biological activity regulators. They are of interest for pharmaceutical, food, and cosmetic applications due to their compartmentalizing nature. Understanding how these structures interact with different interfaces is essential for their use in biomedical devices for drug delivery and analysis. These non-lamellar interfacial layers can entrap functional biomolecules that respond to lipid curvature and confinement.
Additional documents
Useful links
There are no additional links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Illustrations

Self-assembled functional reversed-phase nonlamellar liquid crystal gel formed around API molecules by the phospholipids
Camurus. (n.d.). Technology. Retrieved June 20, 2024, from https://www.camurus.com/science/technology/.
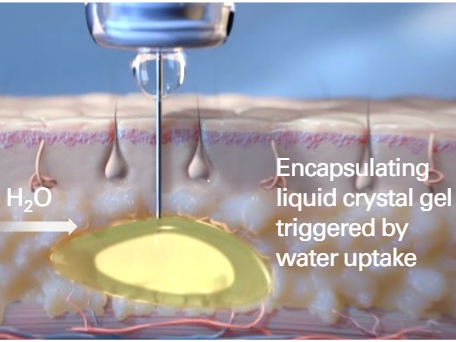
Subcutaneous Administration of FluidCrystal prefilled Injection and the initiation of the encapsulation
Camurus. (2023). Camurus Annual Report 2023. Retrieved from https://www.camurus.com/files/Main/13456/3952729/camurus-annual-report-2023.pdf
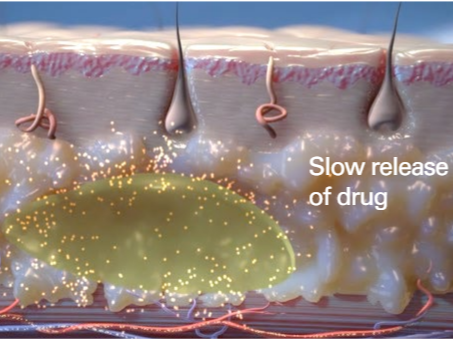
Initial Burst of the API into the body from the FluidCrystal lipid lamellar structure
Camurus. (2023). Camurus Annual Report 2023. Retrieved from https://www.camurus.com/files/Main/13456/3952729/camurus-annual-report-2023.pdf