
|
Developed by 

|
Supported by 

|
In-situ Microimplants
Developer(s)

|
ROVI Originator
https://www.rovi.es/
Spain Laboratorios Farmacéuticos ROVI, founded in 1946 in Madrid, Spain, is a prominent global player in the pharmaceutical industry. Initially established as a domestic company, ROVI has evolved into an international leader in the research, development, manufacturing, and commercialization of both small molecules and biologics using in situ microimplants. |
Sponsor(s)
|
No sponsor indicated |
Partnerships

|
Moderna, Inc. https://www.modernatx.com/en-GB |

|
Insud Pharma, Inc https://www.insudpharma.com/en/ |

|
Center for Industrial Technological Development - CDTI http://www.cdti.es/en/node/889 |
Technology information
Type of technology
Polymer-based in-situ implant
Administration route
Intramuscular
Development state and regulatory approval
Risperidone
Phase III
Approved by the EMA under the brand name OKEDI on 14/02/2022 and approved by the FDA under the brand name RISVAN on 02 April 2024.
Description
Insitu implant (ISM) technology addresses common limitations associated with traditional prolonged-release oral and injectable formulations. This innovative approach utilizes a solid, biodegradable polymer composed of L-lactide and glycolide monomers. By employing a polymeric matrix, ISM addresses several critical limitations, including complex administration procedures, low encapsulation efficiency, and compromised stability of active substances. Furthermore, this technology enables precise control over the initial release of the drug, resulting in consistent therapeutic efficacy.
Technology highlight
The in situ implant is a monthly intramuscular injectable no-particulate solid implant composed of a biodegradable copolymer matrix of dimethylaminomethacrylate and other acrylate monomers, exhibiting enhanced encapsulation efficiency and improved stability of the API. The molecular weight of the copolymers exceeds 15 kDa, contributing to the controlled degradation and release profile. Upon injection, the formulation forms a solid depot at the site, enabling the gradual and sustained drug release over several weeks to months. The API particles are maintained within a size range of 10 to 225 microns, optimizing the balance between immediate and sustained release kinetics.
Technology main components
(i) Biocompatible polymer ( Copolymer of poly ( lactic acid ) and poly ( lactic acid - co - glycolic acid ) (ii) Water miscible solvent ( Dipole moment is in between 3.5-4.5D) (iii) API dissolved in a miscible solvent
Information on the raw materials sourcing, availability and anticipated price
The price of OKEDI (Risperidone ISM) is £222.64/vial in the United Kingdom and its equivalent in EU states.
Delivery device(s)
No delivery device
APIs compatibility profile
API desired features
Water-soluble molecules
Min: 1
Max: 2
Unit: mg/mL
Drug hereby includes the API and/or a metabolite or a prodrug thereof
Small molecules
Targeted drugs for ISM formulation are water-soluble antipsychotic drugs including but not limited to paliperidone, risperidone, olanzapine, opioids like fentanyl, and aromatase inhibitors including but not limited to letrozole and anastrozole.
Additional solubility data
The water-miscible solvent utilized for dissolving the active pharmaceutical ingredient (API) should exhibit a dipole moment in the range of approximately 3.7 to 4.5 Debye (D) and a dielectric constant between 30 and 50.
Additional stability data
The viscosity of the ISM is targeted to fall within the range of 0.50 to 4.0 Pascal-seconds (Pa·s).
API loading: Maximum drug quantity to be loaded
30-50 wt%
API co-administration
1 single API :
LogP
Min: -1
Max: 3
Only hydrophilic and partial hydrophilic drugs are suitable
Scale-up and manufacturing prospects
Scale-up prospects
ROVI Farmaceuticos has expanded its injectable manufacturing capacity with three new plants, producing 250 million syringes and 160 million vials annually.
Tentative equipment list for manufacturing
Not provided
Manufacturing
ISM manufacturing unit is in accordance with ISO 140001:2015 for the production of pharmaceutical specialties and health products in low-volume injectable forms and suppositories. The manufacturing process for these products involves the following steps: 1) Mixing lactic acid and glycolic acid monomeric polymers at a range from 48:52 to 100:0. 2) Mixing the mixture with API dissolved in a water-miscible solvent. 3) Expose the mixture to Beta-radiation (5 - 23 KGy). 4) Sterilize the solvent by filtering it through a medium having a pore size of 0.22 microns or less. 5) Reconstitution process.
Specific analytical instrument required for characterization of formulation
Not provided
Clinical trials
PRISMA-1
Identifier
NCT01788774
Link
https://clinicaltrials.gov/study/NCT01788774
Phase
Phase I
Status
Completed
Sponsor
Rovi Pharmaceuticals Laboratories
More details
This clinical trial is designed to evaluate different dosages of risperidone ISM, a new long-acting injectable form.
Purpose
Pharmacokinetic, Safety, and Tolerability Study of Risperidone ISM® at Different Dose Strengths
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2013-04-01
Anticipated Date of Last Follow-up
2023-10-19
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2014-02-01
Actual Completion Date
2014-02-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Capable of providing informed consent. * Male or female aged ≥ 18 years to \< 65 years * Current diagnosis of schizophrenia or schizoaffective disorder, according to the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders Clinical Trials (SCID-CT) or the DSM-IV-TR * Medically stable over the last month, and psychiatrically stable without significant symptom exacerbation over the last three months based on the investigator's judgment * Score of ≤ 4 (moderately ill) on the Clinical Global Impression - Severity of Illness (CGI-S) * If a sexually active female of childbearing potential, using a medically accepted contraceptive method. Exclusion Criteria: * Presence of an uncontrolled, unstable, clinically significant medical condition
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
36
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Not provided
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
RPV-RISP-2016-02
Identifier
NCT03527186
Link
https://clinicaltrials.gov/study/NCT03527186
Phase
Phase I
Status
Completed
Sponsor
Rovi Pharmaceuticals Laboratories
More details
This is an open-label, one sequence study to evaluate the steady-state comparative bioavailability of 100 mg Risperidone ISM® injectable every 4 weeks compared to once daily 4 mg oral risperidone in subjects with schizophrenia stabilized on oral risperidone treatment.
Purpose
Comparative Bioavailability of Risperidone
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2018-07-09
Anticipated Date of Last Follow-up
2021-12-01
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2019-03-23
Actual Completion Date
2019-04-06
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Subjects will be considered eligible to participate in this study if each one of the following inclusion criteria is satisfied at screening (or at baseline when specified): 1. Male or female aged ≥18 and \<65 years with a body mass index (BMI) of ≥17 kg/m2 but ≤35 kg/m2 2. Current diagnosis of schizophrenia, according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria 3. Outpatient; not hospitalized for worsening of schizophrenia within the last 3 months (hospitalization for social management within this time period is acceptable) 4. Medically stable over the last month and psychiatrically stable without significant symptom exacerbation over the last 3 months based on the investigator's judgement 5. On oral risperidone 4 mg daily dose.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
81
Allocation
Not provided
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
ROV-RISP-2020-01
Identifier
NCT05179525
Link
https://clinicaltrials.gov/study/NCT05179525
Phase
Phase I
Status
Completed
Sponsor
Rovi Pharmaceuticals Laboratories
More details
This is an Open-Label, One-Sequence Study to Evaluate the Steady- State Comparative Bioavailability of Intramuscular Risperidone ISM® and EU Risperdal® (Sourced From Germany).
Purpose
Comparative Bioavailability of Risperidone.
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-03-09
Anticipated Date of Last Follow-up
2021-12-16
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2021-09-17
Actual Completion Date
2021-09-17
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: Subjects will be considered eligible to participate in this study if each one of the following inclusion criteria is satisfied at screening (or at baseline when specified): 1. Male or female aged ≥18 and \<65 years with a body mass index (BMI) of ≥17 kg/m2 but ≤35 kg/m2 2. Current diagnosis of schizophrenia, according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria 3. Outpatient; not hospitalized for worsening of schizophrenia within the last 3 months (hospitalization for social management within this time period is acceptable) 4. Medically stable over the last month and psychiatrically stable without significant symptom exacerbation over the last 3 months based on the investigator's judgement
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
80
Allocation
Not provided
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Not provided
Studied LA-formulation(s)
Not provided
Studied route(s) of administration
Not provided
Use case
Not provided
Key resources
PRISMA-2
Identifier
NCT02086786
Link
https://clinicaltrials.gov/study/NCT02086786
Phase
Phase II
Status
Completed
Sponsor
Rovi Pharmaceuticals Laboratories
More details
To characterize the pharmacokinetics (PK) of the injectable intramuscular (IM) long-acting formulation (in situ microparticle, ISM) of risperidone over four IM injections in the gluteal and deltoid muscle at 28-day intervals and at one dose strength (75 mg) in patients with schizophrenia.
Purpose
Pharmacokinetics and Tolerability Study of Risperidone ISM® in Schizophrenia
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2014-03-01
Anticipated Date of Last Follow-up
2017-06-14
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2015-03-01
Actual Completion Date
2015-03-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Capable of providing informed consent. 2. Male or female aged ≥18 years to ≤65 years. 3. Current diagnosis of schizophrenia, according to Diagnostic and Statistical Manual 4. Body mass index (BMI) ≥17 kg/m2 but ≤35 kg/m2. 5. Medically stable over the last month, and psychiatrically stable 6. On oral stable dosage of risperidone ≥4 mg daily as maintenance therapy. 7. Total score ≤70 on the Positive and Negative Syndrome Scale. 8. Using a medically accepted contraceptive method 9. Agrees to washout all prohibited medications prior to baseline (day -1) Exclusion Criteria: 1. Informed consent obtained from a third party. 2. Prisoners or patients who are compulsorily detained. 3. Females who are breast-feeding and/or who have a positive pregnancy test. 4. Presence of a
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
70
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
PRISMA-3
Identifier
NCT03160521
Link
https://clinicaltrials.gov/study/NCT03160521
Phase
Phase III
Status
Completed
Sponsor
Rovi Pharmaceuticals Laboratories
More details
This is a multicenter, randomized, double-blind, placebo-controlled study designed to evaluate the efficacy and safety of intramuscular (IM) injections of Risperidone ISM® (75 or 100 mg) or placebo, in patients with acute exacerbation of schizophrenia.
Purpose
Study to Evaluate the Efficacy and Safety of Risperidone in Situ Microparticle (ISM)® in Patients With Acute Schizophrenia
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2017-06-02
Anticipated Date of Last Follow-up
2022-02-07
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2018-12-17
Actual Completion Date
2018-12-17
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: To be eligible for enrolment into the study, each patient must meet all of the following criteria at screening: 1. Capable of providing informed consent 1. A signed informed consent form must be provided before any study assessments are performed 2. Patients must be fluent in the language that is spoken by the investigator and the study site staff (including raters) and must be able to read and understand the words in which the informed consent is written 2. Age ≥ 18 and ≤ 65 years 3. Body mass index 18.5 to 40.0 kg/m2 (inclusive) 4. Current diagnosis of schizophrenia, according to the Diagnostic and
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
438
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
PRISMA-3_OLE
Identifier
NCT03870880
Link
https://clinicaltrials.gov/study/NCT03870880
Phase
Phase III
Status
Completed
Sponsor
Rovi Pharmaceuticals Laboratories
More details
This is the long-term open label extension (OLE) of the study PRISMA-3 (NCT03160521). Those patients who complete participation in the main segment of the study (double blind) together with other clinically stable not previously enrolled (de novo patients) may opt to participate in this extension segment, where they will receive active Risperidone ISM® (75 mg or 100 mg)under open-label conditions every four weeks for approximately 12 months.
Purpose
Study to Evaluate the Efficacy and Safety of Risperidone ISM® in Patients With Acute Schizophrenia: Open Label Extension
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2017-08-25
Anticipated Date of Last Follow-up
2022-02-24
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2020-01-08
Actual Completion Date
2020-01-08
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Participation in the open-label extension segment of the study PRISMA-3 is optional, and patients who complete participation in the main segment of the study (double blind segment of PRISMA-3, NCT03160521) may opt to not participate. Patients who are interested in participating must meet all eligibility criteria in order to enter into the extension segment. Inclusion Criteria (Rollover patients): To be eligible for entry into the extension segment of the study PRISMA-3, a patient must meet all of the following criteria at the extension baseline time point (immediately upon completion of the end-of-treatment visit assessments and procedures for the main part of the study): 1. Has completed scheduled participation in the double blind segment of the study PRISMA-3, through to the end of th
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
215
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
HMU
Identifier
NCT01630148
Link
https://clinicaltrials.gov/study/NCT01630148
Phase
Not provided
Status
Completed
Sponsor
Hawler Medical University
More details
The use of prophylaxis for venous thromboembolism (VTE) remains grossly underused for women who undergo gynecologic surgery for benign conditions world wide and especially in developing countries including our region. Having a research in our locality for the first time might raise awareness of the importance of VTE prophylaxis.
Purpose
Bemiparin as a Thromboprophylaxis After Gynaecological Surgeries
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2012-07-01
Anticipated Date of Last Follow-up
2024-09-12
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2014-03-01
Actual Completion Date
2014-03-01
Studied populations
Age Cohort
- Children
- Adults
- Older Adults
Genders
- Female
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * female undergoing Benign gynecological surgeries. * Having moderate,high and very high risk factors for venous thromboembolism. * No contraindications for the use of Heparin. Exclusion Criteria: * Having mild risk factors for thromboembolism. * Active vaginal bleeding. * Thrombocytopaenia. * any patient who is already on anticoagulant. * Sever renal or Liver diseases.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
774
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Single blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
RESHAPE
Identifier
NCT05480046
Link
https://clinicaltrials.gov/study/NCT05480046
Phase
Phase I
Status
Recruiting
Sponsor
Rovi Pharmaceuticals Laboratories
More details
This is a prospective, non-interventional, multicentre study designed to collect information about the effectiveness, safety and tolerability of Risperidone ISM in patients diagnosed with schizophrenia who are suffering an acute exacerbation, according to routine clinical practice.
Purpose
Non-interventional Study of Risperidone ISM® in Schizophrenia Patients Hospitalised Due to a Relapse
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-10-18
Anticipated Date of Last Follow-up
2024-09-09
Estimated Primary Completion Date
2024-12-01
Estimated Completion Date
2024-12-01
Actual Primary Completion Date
2015-12-01
Actual Completion Date
2015-12-01
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: 1. Subject provides informed consent (approved by an Independent Ethical Committee (IEC)) before any study specific evaluation is performed. 2. Subject is between the ages of 18 and 45 years, inclusive. 3. All female subjects must have a negative pregnancy test at Screening and upon check-in to the clinic. 4. Women of childbearing potential must use or have used one of the following acceptable birth control methods: Surgical sterilization (bilateral tubal ligation, hysterectomy, bilateral oophorectomy) for at least 6 months before the first dose of study drug;Intrauterine device in place for at least 3 months before the first dose of study drug; or barrier method (condom, diaphragm) for at least 21 days before the first dose of study drug and throughout the study.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
1200
Allocation
Randomized
Intervention model
Cross-over assignment
Intervention model description
Not provided
Masking
Triple-blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
QUARTZ
Identifier
NCT06276361
Link
https://clinicaltrials.gov/study/NCT06276361
Phase
Phase I
Status
Recruiting
Sponsor
Rovi Pharmaceuticals Laboratories
More details
This is a single ascending dose phase 1 study to evaluate the pharmacokinetics (PK), safety, and tolerability of a single intramuscular (IM) injection of quarterly Risperidone (QUAR) for different formulations and dose strengths in participants with schizophrenia.
Purpose
Pharmacokinetics, Safety and Tolerability of Different Formulations and Dose Strengths of Quarterly Risperidone (QUAR) in Patients With Schizophrenia
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-09-26
Anticipated Date of Last Follow-up
2024-07-08
Estimated Primary Completion Date
2026-05-01
Estimated Completion Date
2026-05-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Capable of providing informed consent. * Male or female aged ≥ 18 years to \< 65 years with BMI ≥17.0 to ≤35.0 kg/m2 * Current diagnosis of schizophrenia, according to the Diagnostic and DSM-5 criteria. * Medically stable over the last month, and psychiatrically stable without significant symptom exacerbation over the last three months based on the investigator's judgment * currently taking oral risperidone as maintenance therapy * Score of ≤ 4 (moderately ill at most) on the Clinical Global Impression - Severity of Illness (CGI-S) * If a sexually active female of childbearing potential, using a medically accepted method of birth control. Exclusion Criteria: * Presence of an uncontrolled, unstable, clinically significant medical condition
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
100
Allocation
Randomized
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
ROV-RISP-2020-01
Identifier
NCT05179525
Link
https://clinicaltrials.gov/study/NCT05179525
Phase
Phase I
Status
Completed
Sponsor
Rovi Pharmaceuticals Laboratories
More details
This is an Open-Label, One-Sequence Study to Evaluate the Steady- State Comparative Bioavailability of Intramuscular Risperidone ISM® and EU Risperdal® (Sourced From Germany).
Purpose
Comparative Bioavailability of Risperidone.
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2021-03-09
Anticipated Date of Last Follow-up
2021-12-16
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2021-09-17
Actual Completion Date
2021-09-17
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: Subjects will be considered eligible to participate in this study if each one of the following inclusion criteria is satisfied at screening (or at baseline when specified): 1. Male or female aged ≥18 and \<65 years with a body mass index (BMI) of ≥17 kg/m2 but ≤35 kg/m2 2. Current diagnosis of schizophrenia, according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria 3. Outpatient; not hospitalized for worsening of schizophrenia within the last 3 months (hospitalization for social management within this time period is acceptable) 4. Medically stable over the last month and psychiatrically stable without significant symptom exacerbation over the last 3 months based on the investigator's judgement
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
80
Allocation
Not provided
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
LEILA-1
Identifier
NCT06315205
Link
https://clinicaltrials.gov/study/NCT06315205
Phase
Phase I
Status
Recruiting
Sponsor
Rovi Pharmaceuticals Laboratories
More details
This is a Phase I, open label, sequential, single ascending dose (SAD) study to evaluate the pharmacokinetic (PK), safety, and tolerability of Letrozole LEBE in healthy post-menopausal women.
Purpose
Evaluation of the Pharmacokinetics, Safety, and Tolerability of IM Letrozole LEBE in Healthy Post-menopausal Women
Interventions
Intervention 1
Intervention 2
Intervention 3
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-07-26
Anticipated Date of Last Follow-up
2024-03-18
Estimated Primary Completion Date
2025-01-01
Estimated Completion Date
2025-01-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Female
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: * Healthy post-menopausal women. * Capable of providing informed consent. * Weight of ≥50 kg and a BMI ≥19 and ≤39 kg/m2. * Subjects should be able to communicate with clinic staff. Exclusion Criteria: * Subjects who have a history of allergy or hypersensitivity to letrozole or any of the inactive ingredients. * Subjects who have a history of galactose intolerance, severe hereditary lactase deficiency glucose-galactose malabsorption. * Subjects who have used estrogen or progesterone hormone replacement therapy, thyroid replacement therapy, oral contraceptives, androgens, luteinizing hormone (LH) releasing hormone analogs, prolactin inhibitors, or antiandrogens within prior to Screening.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
90
Allocation
Not provided
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
ROV-LET-2017-01
Identifier
NCT03401320
Link
https://clinicaltrials.gov/study/NCT03401320
Phase
Phase I
Status
Not provided
Sponsor
Rovi Pharmaceuticals Laboratories
More details
This is a Phase I, open label, dose escalation study designed to evaluate the pharmacokinetics, safety, and tolerability of single intramuscular injections of Letrozole ISM® at different strengths in voluntary healthy post menopausal women
Purpose
Evaluation of IM Letrozole ISM® Pharmacokinetics, Safety, and Tolerability in Healthy Post-menopausal Women (LISA-1)
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2017-11-06
Anticipated Date of Last Follow-up
2024-03-22
Estimated Primary Completion Date
2024-05-01
Estimated Completion Date
2024-05-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- Female
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: 1. Healthy post-menopausal women, ≥ 18 and ≤ 75 years of age, who have achieved complete menopause, either natural or surgical, and amenorrhea, and have not been on hormone replacement therapy in the last 3 months. 2. Post-menopausal subjects should have absence of menses for 1 year, and oophorectomized subjects should have absence of menses for at least 6 weeks. For oophorectomized subjects and subjects who have had a hysterectomy, a surgical pathology report documenting the absence of malignant disease is required. In addition, for oophorectomized subjects an operative report documenting bilateral oophorectomy is required. 3. Baseline follicle-stimulating hormone (FSH) and 17β-estradiol plasma levels should be consistent with the post-menopausal status of the subject
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
120
Allocation
Not provided
Intervention model
Sequential assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
Excipients
Proprietary excipients used
No proprietary excipient used
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
No novel excipient or existing excipient used
Residual solvents used
No residual solvent used
Additional features
Other features of the technology
- Biodegradable
- Drug-eluting
- Monolithic
- Removable
- At least 1 year shelf life
- Needs insertion kit
Release properties
ISM provides a sustained release of API and achieves therapeutic plasma levels within the first day after a single dose of API. The ISM provides a sustained release throughout the 4-week dosing period over multiple intramuscular injections.
Injectability
Intramuscular ISM injections are prefilled injections with 20G and 21G needles. The injection kit contains a powder-prefilled syringe, a solvent-prefilled syringe, and two sterile safety hypodermic needles: a 20G needle (for gluteus muscle) and a 21G needle (deltoid muscle). Phase I studies revealed a 15% incidence of injection site erythema and a 7.5% incidence of injection site induration, which healthcare professionals should carefully consider.
Safety
Phase I studies of Risperidone ISM demonstrated that the most frequent adverse event was hyperprolactinemia, occurring in 54% of participants. A total of 13 serious adverse events (SAEs) were reported, including dystonia.
Stability
The ISM has a non-degradable/ non-erodible that provides structural stability for the device regardless of the pH of a surrounding aqueous environment.
Storage conditions and cold-chain related features
Not provided
Potential application(s)
Therapeutic area(s)
Use case(s)
Not provided
Use of technology
Ease of administration
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
Frequency of administration
Weekly, Monthly
User acceptance
Not provided
Targeted user groups
Age Cohort- Adults
- Older Adults
- All
Pregnant individuals
Unspecified
Lactating individuals
Unspecified
Healthy individuals
No
Comment
Not provided
Potential associated API(s)
Paliperidone
Class(es)
Type D2 Dopamine receptor antagonist
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Schizophrenia
Foreseen user group
Not provided
Foreseen duration between application(s)
Not provided
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Risperidone
Class(es)
alpha-1 (α1), alpha-2 (α2), and histamine (H1) receptor antagonist
Development stage
Phase III
Clinical trial number(s)
NCT03160521
Foreseen/approved indication(s)
Schizophrenia
Foreseen user group
18-65 aged adults with Schizophrenia
Foreseen duration between application(s)
Once monthly
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Approved by the EMA under the brand name OKEDI on 14/02/2022 and approved by the FDA under the brand name RISVAN on 02 April 2024.
letrozole
Class(es)
Aromatase Inhibitors
Development stage
Phase I
Clinical trial number(s)
NCT03401320; NCT06315205
Foreseen/approved indication(s)
Breast Cancer
Foreseen user group
Post Menopausal women
Foreseen duration between application(s)
Not provided
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Patent info
Description
Procedure for the filling of solids in pharmaceutical containers and the sealing thereof under sterile conditions
Brief description
A sterile procedure for the filing of solids into pharmaceutical containers and the sealing thereof under sterile conditions is provided. Exemplary containers include syringes, vials, capsules, ampoules, single-dose devices or cartridges. The containers can be filled with powder, granules, nanoparticles or microparticles. After sealing, the containers are airtight. More specifically, the procedure minimizes adherence of those solids to the interior surfaces of the containers during the filling and sealing steps, thus ensuring airtightness of the seal and precision of the weight of the solid dispensed into the containers.
Representative patent
US11987410B2
Category
Not provided
Patent holder
Laboratorios Farmaceuticos Rovi SA
Exclusivity
Not provided
Expiration date
May 23, 2040
Status
Active
Description
Injectable composition with aromatase inhibitor
Brief description
The present invention provides a composition suitable for forming an intramuscular implant. It comprises a biodegradable thermoplastic polymer of polylactic acid (PLA), DMSO and an aromatase inhibitor compound. The invention also provides a kit suitable for the in situ formation of the composition and its use as a medicine for treating cancer, especially breast cancer.
Representative patent
US11918682B2
Category
Composition of the formulation
Patent holder
Laboratorios Farmaceuticos Rovi SA
Exclusivity
Not provided
Expiration date
April 16, 2032
Status
Active
Description
Antipsychotic injectable depot composition
Brief description
The present invention is directed to a composition that can be used to deliver an antipsychotic drug such as risperidone as an injectable in-situ forming biodegradable implant for extended release providing therapeutic plasma levels from the first day. The composition is in the form of drug suspension on a biodegradable and biocompatible copolymer or copolymers solution using water miscible solvents that is administered in liquid form. Once the composition contacts the body fluids, the polymer matrix hardens retaining the drug, forming a solid or semisolid implant that releases the drug in a continuous manner. Therapeutic plasma levels of the drug can be achieved since the first day up to at least 14 days or more even up to at least four weeks.
Representative patent
US10182982B2
Category
Composition of the formulation
Patent holder
Laboratorios Farmaceuticos Rovi SA
Exclusivity
Not provided
Expiration date
May 31, 2031
Status
Active
Description
Risperidone or paliperidone implant formulation
Brief description
The present invention is directed to an injectable intramuscular depot composition suitable for forming an in situ solid implant in a body, comprising a drug which is risperidone and/or paliperidone or any pharmaceutically acceptable salt thereof in any combination, a biocompatible copolymer based on lactic and glycolic acid having a monomer ratio of lactic to glycolic acid of about 50:50 and DMSO solvent, wherein the composition releases the drug with an immediate onset of action and continuously for at least 4 weeks and wherein the composition has a pharmacokinetic profile in vivo that makes it suitable to be administered each 4 weeks or even longer periods.
Representative patent
US11007139B2
Category
Formulation
Patent holder
Laboratorios Farmaceuticos Rovi SA
Exclusivity
Not provided
Expiration date
May 31, 2031
Status
Active
Description
Methods for the Preparation of Injectable Depot Compositions
Brief description
Injectable depot compositions, comprising a biocompatible polymer which is a polymer or copolymer based on lactic acid and/or lactic acid plus glycolic acid having a monomer ratio of lactic to glycolic acid in the range from 48:52 to 100:0, a water-miscible solvent having a dipole moment of about 3.7-4.5 D and a dielectric constant of between 30 and 50, and a drug, were found suitable for forming in-situ biodegradable implants which can evoke therapeutic drug plasma levels from the first day and for at least 14 days.
Representative patent
US20180318208A1
Category
Not provided
Patent holder
Laboratorios Farmaceuticos Rovi SA
Exclusivity
Not provided
Expiration date
May 31, 2024
Status
Active
Supporting material
Publications
<p><span style="color: rgb(33, 33, 33);">Messer, T., Bernardo, M., Anta, L., & Martínez-González, J. (2024). Risperidone ISM®: review and update of its usefulness in all phases of schizophrenia. </span><em style="color: rgb(33, 33, 33);">Therapeutic advances in psychopharmacology</em><span style="color: rgb(33, 33, 33);">, </span><em style="color: rgb(33, 33, 33);">14</em><span style="color: rgb(33, 33, 33);">, 20451253241280046. </span><a href="https://doi.org/10.1177/20451253241280046" rel="noopener noreferrer" target="_blank" style="color: rgb(33, 33, 33);">https://doi.org/10.1177/20451253241280046</a></p>
One of the most important challenges in the management of patients with schizophrenia is to ensure adherence to antipsychotic treatment. The contribution of long-acting injectables (LAI) is undeniable in this matter, but there are still some unmet medical needs not covered by these drugs (e.g. quick onset of action for patients with acute exacerbation of schizophrenia). This article summarises the pharmacokinetics, efficacy and safety of Risperidone ISM (in situ microparticles). The aim of this review is to provide information about the potential uses of this new LAI formulation of risperidone for the treatment of schizophrenia, contextualising and diving into the published evidence. Risperidone ISM shows a rapid release which allows achieving within 12 h risperidone active moiety levels similar to those observed in the steady-state for oral risperidone treatment, achieving a mean average concentration of 38.63 ng/mL. The plasma concentration of active moiety achieved by Risperidone ISM comes with a predictable dopamine D2 receptor occupancy above 65% throughout the 28-day dosing period, which is accepted as a threshold for the efficacy of the antipsychotic treatment. This can be associated with the positive efficacy findings throughout its clinical development. In the short term, it provides an early and progressive reduction of symptoms in adult patients with acute exacerbation of schizophrenia without the need for loading doses or oral risperidone supplementation, which could contribute to reinforcing the therapeutic alliance between the patient and the psychiatrist. In addition, long-term treatment was effective, safe and well tolerated regardless of the initial disease severity or whether patients were previously treated with Risperidone ISM during an acute exacerbation or switched from stable doses of oral risperidone. Improvement and maintenance of personal and social functioning and health-related quality of life were observed in each setting, respectively. All these findings endorse Risperidone ISM as a useful and valuable treatment for the acute and maintenance management of patients with schizophrenia.
<p><span style="color: rgb(33, 33, 33);">Laveille, C., Snoeck, E., Ochoa Díaz de Monasterioguren, L., Martínez-González, J., Llaudó, J., Anta, L., & Gutierro, I. (2024). Development of a population pharmacokinetic model for the novel long-acting injectable antipsychotic risperidone ISM®. </span><em style="color: rgb(33, 33, 33);">British journal of clinical pharmacology</em><span style="color: rgb(33, 33, 33);">, </span><em style="color: rgb(33, 33, 33);">90</em><span style="color: rgb(33, 33, 33);">(9), 2256–2270. </span><a href="https://doi.org/10.1111/bcp.16115" rel="noopener noreferrer" target="_blank" style="color: rgb(33, 33, 33);">https://doi.org/10.1111/bcp.16115</a></p>
The final model adequately described the pharmacokinetics of 6288 active moiety concentrations in 17 healthy volunteers and 430 patients with schizophrenia. This one-compartment disposition model had a complex absorption process, combining a small amount immediately entering the central active moiety compartment, two first-order absorption processes and a combined zero-order and first order process, with first-order elimination from the central compartment. Significant covariates on CL40 were BMI and sex. Goodness-of-fit (GOF) plots and visual predictive checks (VPC) confirmed acceptable description of the data.
<p><span style="color: rgb(33, 33, 33);">Anta, L., Llaudó, J., Ayani, I., Martínez, J., Litman, R. E., & Gutierro, I. (2018). A phase II study to evaluate the pharmacokinetics, safety, and tolerability of Risperidone ISM multiple intramuscular injections once every 4 weeks in patients with schizophrenia. </span><em style="color: rgb(33, 33, 33);">International clinical psychopharmacology</em><span style="color: rgb(33, 33, 33);">, </span><em style="color: rgb(33, 33, 33);">33</em><span style="color: rgb(33, 33, 33);">(2), 79–87. </span><a href="https://doi.org/10.1097/YIC.0000000000000203" rel="noopener noreferrer" target="_blank" style="color: rgb(33, 33, 33);">https://doi.org/10.1097/YIC.0000000000000203</a></p>
This study characterized the pharmacokinetics, safety, and tolerability of Risperidone ISM, a new long-acting intramuscular formulation, for monthly administration without oral supplementation. Patients with schizophrenia received multiple intramuscular injections of 75 mg in the gluteal or deltoid muscle at 28-day intervals. Of the 70 randomized patients, 67 received at least one dose of study medication. The mean Cmax of the active moiety was achieved 24-48 h (tmax) after each administration, regardless of injection site. They ranged over four consecutive doses from 39.6 to 53.2 and 54.1 to 61 ng/ml, when given in gluteal or deltoid muscle, respectively. Active moiety achieved therapeutic levels by 2 h after dose, and the levels were maintained throughout the 4-week dosing period. No significant changes across the study were observed on either Positive and Negative Syndrome Scale or Extrapyramidal Symptoms Scale. Overall, 63 (94%) patients experienced at least one treatment-emergent adverse event (TEAE). One serious TEAE (dystonia) was related to study treatment. The most frequently reported TEAEs were hyperprolactinaemia (57.7%) and injection site pain (32.8%). Risperidone ISM achieved therapeutic levels from the first hours after drug administration and provided a sustained release throughout the 4-week dosing period over multiple intramuscular injections and was found to be safe and well tolerated.
Additional documents
No documents were uploaded
Useful links
There are no additional links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Illustrations

Insitu Microimplant illustration by ROVI
https://rovi.es/
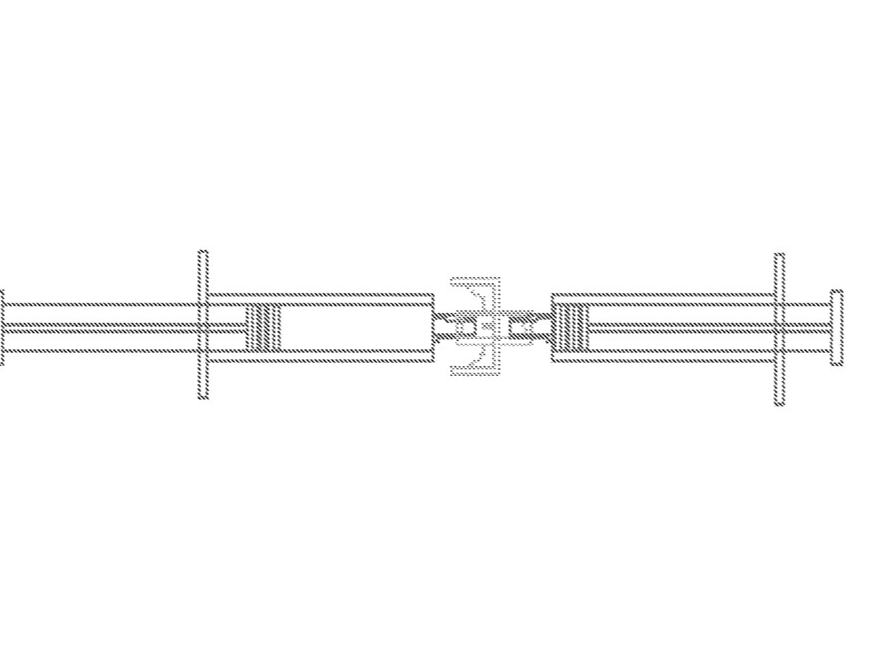
Exemplary embodiment of syringes suitable for administering the ISM injectable composition .
https://patentimages.storage.googleapis.com/58/1f/46/a896079d169a11/US20180318208A1.pdf
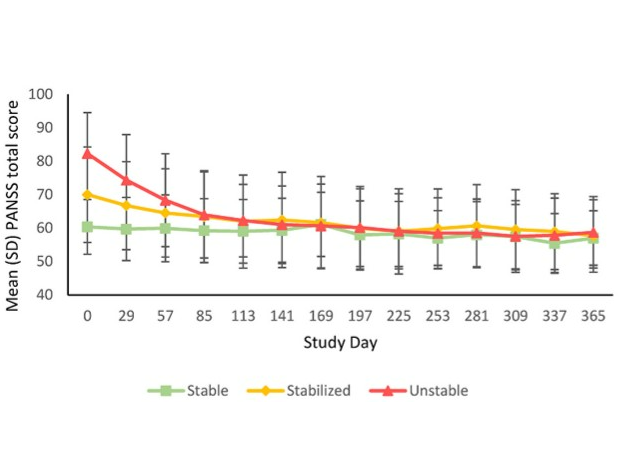
Mean (SD) PANSS total score at each time point in Unstable, Stabilized and Stable patients treated with monthly Risperidone ISM® (pooled 75 and 100 mg).
Filts, Y., Litman, R. E., Martínez, J., Anta, L., Naber, D., & Correll, C. U. (2022). Long-term efficacy and safety of once-monthly Risperidone ISM® in the treatment of schizophrenia: Results from a 1
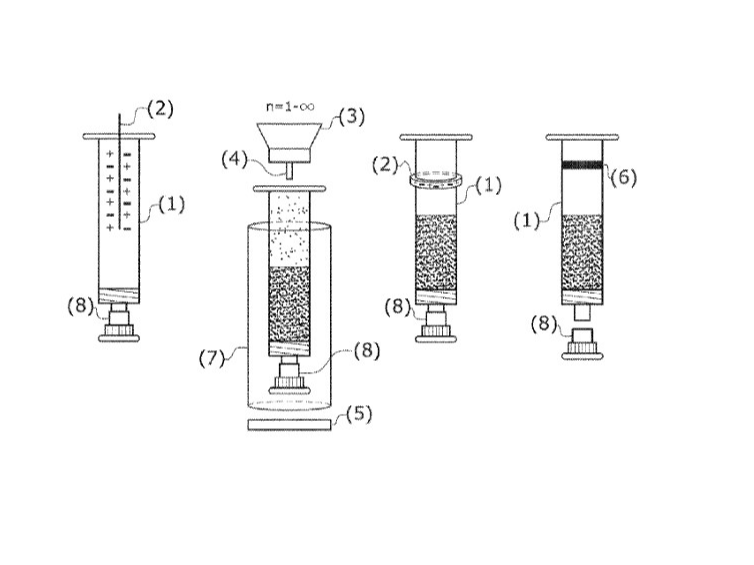
Exemplary embodiment of the patented injection kit of ISM and its step-wise usage procedure
https://patentimages.storage.googleapis.com/0f/c9/bc/7d836740c18f54/US11987410.pdf