
|
Developed by 

|
Supported by 

|
LYNX
Based on public informationDeveloper(s)

|
Lyndra Therapeutics https://lyndra.com/United States of America In 2015, Lyndra Therapeutics was founded by Robert Langer to create a pipeline of long-acting drugs. Since its creation, Lyndra has made significant progress, developing 25 medicines in the lab, finishing 12 clinical studies, and establishing a proof of concept for weekly oral dosing in 5 therapeutic areas, all of which validate the viability of its platform with various APIs. |
Sponsor(s)
|
No sponsor indicated |
Partnerships

|
National Institute of Health https://www.nih.gov |

|
Bill & Melinda Gates Foundation https://www.gatesfoundation.org |

|
Gilead Sciences, Inc. https://www.gilead.com |

|
Abbvie Pharmaceuticals https://www.abbvie.co.uk |
Technology information
Type of technology
Oral solid form
Administration route
Oral
Development state and regulatory approval
Risperidone
Phase III
IND application was approved for LYN-005 by US FDA on 2020
Description
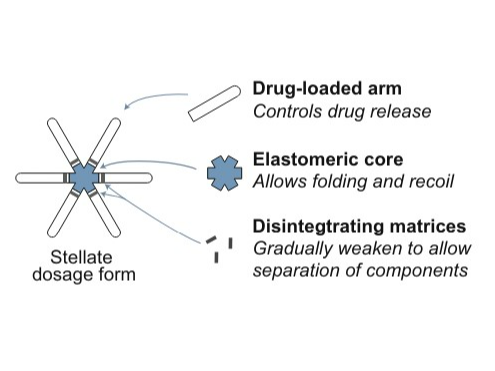
Lynx is an oral drug delivery system that releases the API slowly over time. It has the potential to decrease the number of times a drug needs to be taken from once a day to once a week. This system consists of a standard size capsule (00EL) with a core elastomer and six drug arms folded inside like a stellate. When the capsule dissolves, this stellate structure extends and lodges in the stomach for a week. It is gastric-resistant system which contains a carrier polymer component linked with one or more coupling polymers. This polymer is responsible for the extended release of the API.
Technology highlight
• LYNX consists of a central core attached with six polymer arms . • Each arm contains a concentrated amount of the API. • The capsule is coated with a proprietary material. • This coating makes it easy to swallow and ensures the capsule remains intact in the oesophagus, preventing premature drug release. • The elastomer material used in the arms of LYNX is porous. • This porosity allows for a slow and steady release of the drug into the system. • As a result, the therapeutic concentration of the API is maintained in the plasma for an extended period. • Thus, LYNX helps reduce the peaks and troughs in drug plasma levels. • The arms are connected to the core using biodegradable linkers. • Once the drug release is complete, these linkers soften and disintegrates.
Technology main components
(i) Carrier polymer (eg: polycaprolactone, polyanhydrides, polyphosphazenes, and polycyanoacrylates); (ii) API; (iii) Release enhancer; (iv) Dispersant (eg: carboxymethylcellulose, hypromellose, magnesium aluminum silicate, CABOSIL M-5P); (v) Solubilizer; (vi) Stabilizer (vii) Capsule coating (eg: Eudragit RS, Dichloromethane)
Information on the raw materials sourcing, availability and anticipated price
Not provided
Delivery device(s)
No delivery device
APIs compatibility profile
API desired features
Not provided
Additional solubility data
Not provided
Additional stability data
Not provided
API loading: Maximum drug quantity to be loaded
50wt%
API co-administration
Not provided
LogP
Not provided
Scale-up and manufacturing prospects
Scale-up prospects
In terms of scale up prospective, Lyndra has begun ramping up new manufacturing operations in Lexington, Massachusetts. The plant began producing materials in April in preparation for the company's Phase II clinical trials, which are slated to begin later this year. In order to meet the demands of both upcoming and ongoing clinical studies as well as potential commercialization, Lyndra keeps growing its manufacturing capacity.
Tentative equipment list for manufacturing
Haake MiniCTW, Twin-screw extruders, triangular cross-section rods, coating pan and dip coater.
Manufacturing
• Initially, three 1-kg batches of a matrix formulation were produced and characterized for performance and stability. • Blends of drug, polymer, and excipients blended by continuous twin screw compounding at 500 g/h. • Blends are formed into triangular cross-section rods and cut to length to form drug arms. • Analysis showed good uniformity in both intra-batch and inter-batch. • The prepared formulation is dip-coated, assembled into stellate dosage forms, and analysed for storage stability.
Specific analytical instrument required for characterization of formulation
HPLC with precolumn derivatization, NMR, X-ray diffraction and UV spectroscopy.
Clinical trials
LYN-014-C-101
Identifier
NCT05251376
Link
https://clinicaltrials.gov/study/NCT05251376
Phase
Phase I
Status
Withdrawn
Sponsor
Lyndra Inc.
More details
A Phase 1, Single Dose, Open-label, Safety, Tolerability, and Pharmacokinetic Study of LYN-014 in Individuals with Opioid Use Disorder Who are Stable on Methadone Therapy
Purpose
Study of LYN-014 in Individuals With Opioid Use Disorder Who Are Stable on Methadone Therapy
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Intervention 5
Countries
Not provided
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2022-02-28
Anticipated Date of Last Follow-up
2023-01-17
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2022-12-19
Actual Completion Date
2022-12-19
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: To be eligible to participate in the study, individuals must meet all the following inclusion criteria at Screening (and at other timepoints, where specified): Male or female aged ≥18 and ≤59 years. Body mass index of ≥18 kg/m2 and ≤33 kg/m2. Moderate or severe OUD according to the DSM-5 criteria. Clinically stable (for at least 6 months) on oral daily methadone therapy at a dose of 80 to 100 mg and have been taking the same dose for at least 3 months, and are stably engaged in a methadone program, confirmed by a methadone provider and defined as (1) demonstrates evidence of regular attendance, (2) has not had problems with missed visits, and (3) consistently demonstrates drug-negative urine samples (except for cannabis). Agree to provide the study site with contact
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
Not provided
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
LYN-163-C-101
Identifier
ACTRN12621001218886
Link
https://anzctr.org.au/Trial/Registration/TrialReview.aspx?id=381955&isReview=true
Phase
Phase I
Status
Not provided
Sponsor
Lyndra Therapeutics, Inc
More details
This single ascending dose study will evaluate the safety, tolerability, pharmacokinetics (PK) of LYN-163in healthy individuals. The PK of ivermectin will be assessed. Data from this study will be a key indicator of feasibility of the product concept and will inform formulation optimization and dose selection for further development. This study will enroll individuals who are in good health. Healthy volunteers are most suitable for providing the initial characterization of the LYN-163safety and PK profile after a single dose.
Purpose
Prevention
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2021-10-15
Actual Start Date
2022-05-26
Anticipated Date of Last Follow-up
2023-11-16
Estimated Primary Completion Date
Not provided
Estimated Completion Date
2023-04-15
Actual Primary Completion Date
2023-02-14
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Adolescents
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
No
Accepts healthy individuals
Yes
Comments about the studied populations
Not provided
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
15
Allocation
Not provided
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Not provided
Use case
Treatment
Key resources
LYN 005-C-301
Identifier
NCT05779241
Link
https://clinicaltrials.gov/study/NCT05779241
Phase
Phase III
Status
Completed
Sponsor
Lyndra Inc.
More details
Lyndra Therapeutics, Inc. is developing LYN-005, a long-acting oral (LAO) capsule (LYNX™ dosage form) of risperidone. This pivotal study (LYN-005-C-301) will evaluate the PK as well as safety and tolerability of multiple administrations of the LYN-005 formulation at two dose levels.
Purpose
Study to Evaluate the Pharmacokinetics (PK) and Safety/Tolerability of Long-Acting Oral LYN-005
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-04-13
Anticipated Date of Last Follow-up
2024-03-11
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2023-11-03
Actual Completion Date
2023-12-01
Studied populations
Age Cohort
- Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Male or female aged ≥18 and ≤64 years. 2. Current diagnosis of schizophrenia or schizoaffective disorder according to Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria as confirmed by the Mini International Neuropsychiatric Interview for Psychotic Disorder Studies (MINI) version 7.0.2. 3. The following psychiatric criteria are to be used to determine participant eligibility: 1. Duration of diagnosis of schizophrenia or schizoaffective disorder of ≥2 years. 2. Outpatient; not hospitalized for worsening of schizophrenia within the last 6 months (partial hospitalization for social management within this time period is acceptable). 3. Medically stable over the last month and psychiatrically stable without significant symptom exacerbation o
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
83
Allocation
Non-randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Not provided
Use case
Treatment
Key resources
Excipients
Proprietary excipients used
No proprietary excipient used
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
No novel excipient or existing excipient used
Residual solvents used
No residual solvent used
Additional features
Other features of the technology
- Biodegradable
- Drug-eluting
- Room temperature storage
- At least 1 year shelf life
Release properties
• The LYNX gastric residence system can release the API for a cumulative four to ten days, achieving near-zero-order drug release over a week. • The release characteristics are mainly based on the eudragit & dichloromethane-coated polymer matrix, which tends to release the API linearly over the first 24h once the surface drug is dissolved. • The dispersant added to the formulation also controls the initial burst release and maintains the percentage of drug release over a week. In addition to that, burst release and release rate can be modified by using varied concentrations of dispersants.
Injectability
Not applicable
Safety
Interim analysis of ongoing clinical trials of LYN-005 (Oral Weekly Risperidone) shows positive results based on the PANSS score in schizophrenia. LYNN-005 is generally safe and well-tolerated.
Stability
LYNX gastric resistance system has an extended shelf life of three years
Storage conditions and cold-chain related features
LYN-005 is meant to be stored at 15–25 °C. The capsules are to be handled carefully to avoid squeezing or crushing.
Potential application(s)
Therapeutic area(s)
Use case(s)
Use of technology
Ease of administration
- Self-administered
Frequency of administration
Weekly, Monthly
User acceptance
Not provided
Targeted user groups
Age Cohort- Adults
- Older Adults
- All
Pregnant individuals
Unspecified
Lactating individuals
Unspecified
Healthy individuals
Yes
Comment
Not provided
Potential associated API(s)
Risperidone
Class(es)
Antipsychotic
Development stage
Phase III
Clinical trial number(s)
NCT04567524
Foreseen/approved indication(s)
Antipsychotic
Foreseen user group
Not provided
Foreseen duration between application(s)
Once weekly
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
IND application was approved for LYN-005 by US FDA on 2020
rosuvastatin
Class(es)
HMG-CoA reductase inhibitor
Development stage
Phase I
Clinical trial number(s)
ACTRN12621000101886
Foreseen/approved indication(s)
Hyperlipidemia
Foreseen user group
Not provided
Foreseen duration between application(s)
Once weekly
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
levomethadone
Class(es)
Drug Abuse
Development stage
Phase I
Clinical trial number(s)
NCT05251376
Foreseen/approved indication(s)
Opioid Use Disorder
Foreseen user group
Not provided
Foreseen duration between application(s)
Once weekly
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Received IND on May 2021 and Fast Track designation from FDA
Ivermectin
Class(es)
Antimalarial
Development stage
Phase I
Clinical trial number(s)
ACTRN12621001218886
Foreseen/approved indication(s)
Malaria infection
Foreseen user group
Not provided
Foreseen duration between application(s)
Once every two weeks
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
memantine
Class(es)
NMDAR antagonist
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Alzheimer's disease
Foreseen user group
Not provided
Foreseen duration between application(s)
Once weekly
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Dolutegravir (DTG)
Class(es)
HIV integrase inhibitor
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
HIV
Foreseen user group
Not provided
Foreseen duration between application(s)
Once a week
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Rilpivirine (RPV)
Class(es)
Non-nucleoside reverse transcriptase inhibitors
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
HIV
Foreseen user group
Not provided
Foreseen duration between application(s)
Once a week
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Cabotegravir (CAB)
Class(es)
HIV integrase inhibitors
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
HIV
Foreseen user group
Not provided
Foreseen duration between application(s)
Once a week
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Opioids
Class(es)
Narcotic analgesic
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Pain Management
Foreseen user group
Not provided
Foreseen duration between application(s)
Once a week
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
naloxone
Class(es)
Drug Abuse
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Opioid dependence
Foreseen user group
Not provided
Foreseen duration between application(s)
Once a week
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Patent info
Description
Gastric residence systems with release rate-modulating films
Brief description
he invention provides gastric residence systems, or components of gastric residence system such as segments or elongate members of gastric residence systems, with release rate- modulating films and methods for making and using such systems. The release rate-modulating films provide good control over release of agents (such as therapeutic, diagnostic, or nutritional agents) present in the gastric residence system. The films also permit higher drug loading in the gastric residence systems and components of gastric residence systems while maintaining good control over release of agents. Some embodiments of the films can provide resistance against burst release of agent upon exposure to alcohol.
Representative patent
WO2018227147
Category
Device
Patent holder
Lyndra Therapeutics
Exclusivity
Not provided
Expiration date
June 8, 2038
Status
Granted: AU, JP, US Pending: CA, EP, CN
Description
Gastric residence systems for sustained release of therapeutics agents and methods of use thereof
Brief description
Gastric residence systems comprise therapeutic agent formulations for sustained gastric release of therapeutic agents as well as methods for using such systems. The systems are by using a dispersant in the formulations, which improves the burst release characteristics and long-term release rate characteristics of the systems. Milling of the therapeutic agent can be performed to prepare agent particles of the desired size.
Representative patent
WO2017070612
Category
Device
Patent holder
Lyndra Therapeutics
Exclusivity
Not provided
Expiration date
October 21, 2036
Status
Granted: AU, CA, JP, US Pending: EP, CN
Description
A multi-armed star shaped gastric residence structure loaded with therapeutic agent
Brief description
Residence structures, systems, and related methods are generally provided. Certain embodiments comprise administering (e.g., orally) a residence structure to a subject (e.g., a patient) such that the residence structure is retained at a location internal to the subject for a particular amount of time (e.g., at least about 24 hours) before being released. The residence structure may be, in some cases, a gastric residence structure. In some embodiments, the structures and systems described herein comprise one or more materials configured for high levels of active substances (e.g., a therapeutic agent) loading, high active substance and/or structure stability in acidic environments, mechanical flexibility and strength in an internal orifice (e.g., gastric cavity), easy passage through the GI.
Representative patent
WO2015191920
Category
Device
Patent holder
MIT; Brigham & Women's Hospital; Tokitae LLC
Exclusivity
patents licensed exclusively to LYNDRA INC. from Massachusetts Institute of Technology and Brigham & Women’s Hospital
Expiration date
June 11, 2035
Status
Granted: AU, BR, CA, CN, EP (BE, CH, DE, FR, GB, LI, LU), HK, IL, JP, KR, MX, NZ, RU, SG, US, ZA
Supporting material
Publications
<p><span style="color: rgb(34, 34, 34);">Kanasty, R., Low, S., Bhise, N., Yang, J., Peeke, E., Schwarz, M., ... & Bellinger, A. M. (2019). A pharmaceutical answer to nonadherence: Once weekly oral memantine for Alzheimer's disease. </span><em style="color: rgb(34, 34, 34);">Journal of controlled release</em><span style="color: rgb(34, 34, 34);">, </span><em style="color: rgb(34, 34, 34);">303</em><span style="color: rgb(34, 34, 34);">, 34-41. </span><a href="https://doi.org/10.1016/j.jconrel.2019.03.022" rel="noopener noreferrer" target="_blank" style="color: rgb(2, 114, 177);">https://doi.org/10.1016/j.jconrel.2019.03.022</a></p>
The formulation of memantine hydrochloride is the first oral dosage form to achieve sustained drug release for a week with near zero-order kinetics and efficient delivery. In the dog model, relative memantine bioavailability approaches 100%, with sustained plasma levels over seven days. A single gastric resistant dosage form achieves an AUC equivalent to seven daily treatments with the marketed daily capsule, with a Cmax no higher than the daily product. This formulation methodology is applicable to many water-soluble drugs and may enable the development of long-acting oral therapies for various conditions.
<p><span style="color: rgb(33, 33, 33);">Kirtane AR, Abouzid O, Minahan D, Bensel T, Hill AL, Selinger C, Bershteyn A, Craig M, Mo SS, Mazdiyasni H, Cleveland C, Rogner J, Lee YL, Booth L, Javid F, Wu SJ, Grant T, Bellinger AM, Nikolic B, Hayward A, Wood L, Eckhoff PA, Nowak MA, Langer R, Traverso G. Development of an oral once-weekly drug delivery system for HIV antiretroviral therapy. Nat Commun. 2018 Jan 9;9(1):2. doi: </span><a href="https://www.nature.com/articles/s41467-017-02294-6" rel="noopener noreferrer" target="_blank" style="color: rgb(33, 33, 33);">10.1038/s41467-017-02294-6</a>.</p>
The efficacy of antiretroviral therapy is significantly compromised by medication non-adherence. Long-acting enteral systems that can ease the burden of daily adherence have not yet been developed. Here we describe an oral dosage form composed of distinct drug-polymer matrices which achieved week-long systemic drug levels of the antiretrovirals dolutegravir, rilpivirine and cabotegravir in a pig. Simulations of viral dynamics and patient adherence patterns indicate that such systems would significantly reduce therapeutic failures and epidemiological modelling suggests that using such an intervention prophylactically could avert hundreds of thousands of new HIV cases. In sum, weekly administration of long-acting antiretrovirals via a novel oral dosage form is a promising intervention to help control the HIV epidemic worldwide.
<p><span style="color: rgb(33, 33, 33);">Foltin, R. W., Zale, S., Sykes, K. A., Nagaraj, N., Scranton, R. E., & Comer, S. D. (2022). A novel long-acting formulation of oral buprenorphine/naloxone produces prolonged decreases in fentanyl self-administration by rhesus monkeys. </span><em style="color: rgb(33, 33, 33);">Drug and alcohol dependence</em><span style="color: rgb(33, 33, 33);">, </span><em style="color: rgb(33, 33, 33);">239</em><span style="color: rgb(33, 33, 33);">, 109599. https://doi.org/10.1016/j.drugalcdep.2022.109599</span></p>
We evaluated the efficacy of this formulation in reducing intravenous (i.v.) fentanyl self-administration by three male and three female rhesus monkeys. Buprenorphine HCl and naloxone HCl were co-formulated using an 11:1 ratio of buprenorphine:naloxone in a controlled-release gastric residence formulation administered in an oral capsule (LYN-013). Naloxone was included to determine the feasibility of combining naloxone with buprenorphine in the formulation as an abuse deterrent. Complete fentanyl dose-response functions were determined during each session. The efficacy of single doses of 56/5, 112/10 and 168/15 mg buprenorphine/naloxone in reducing fentanyl self-administration was examined over 13 days. LYN-013 significantly decreased the rate of responding for fentanyl for 3 days and significantly reduced total intake of fentanyl for 8 days. Time to maximal buprenorphine levels (Tmax) ranged between 56 and 68 h for all 3 doses. The maximal buprenorphine level (Cmax) following 168 mg was 2.3 ng/ml which was significantly greater that those observed for 56 mg (1.22 ng/ml) and 112 mg (1.35 ng/ml). Finally, the area-under-curves (AUCtau) were buprenorphine dose-dependently increased from 88 to 127-265 h*ng/ml. There were no signs of non-specific changes in behavior.
Additional documents
No documents were uploaded
Useful links
There are no additional links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Illustrations

Drug-loaded arms control drug release, flexible elastomeric cores allow folding into a capsule and deployment in the stomach, disintegrating matrices control breakdown and passage out of the stomach
Kanasty, R., Low, S., Bhise, N., Yang, J., Peeke, E., Schwarz, M., ... & Bellinger, A. M. (2019). A pharmaceutical answer to nonadherence: Once weekly oral memantine for Alzheimer's disease.