
|
Developed by 

|
Supported by 

|
Mesoporous silica nanoparticles (MSNP)
Developer(s)

|
University of California Originator
https://regents.universityofcalifornia.edu/
United States of America The University of California (UC), established in 1868, is a renowned public research institution comprising 10 campuses, governed by the Board of Regents, which oversees its strategic direction. With a legacy of academic excellence and innovation, UC has pioneered advancements across diverse fields, including mesoporous nanoparticle technology for precision drug delivery and targeted therapies. |
Sponsor(s)
|
No sponsor indicated |
Partnerships

|
Merrimack Pharmaceuticals, Inc. https://www.merrimack.com/ |
|
Washinton University in St.Louis https://medicine.washu.edu/ |

|
National Health Research Institutes https://nicr.nhri.edu.tw/en/ |

|
National Cheng Kung University Hospital https://web.hosp.ncku.edu.tw/nckm/english/index.aspx |
Technology information
Type of technology
Silica based nanoparticles
Administration route
Oral, Subcutaneous, Intramuscular, Intravenous, intraarterial, intracerebral, intrathecal, intranasal
Development state and regulatory approval
irinotecan
Phase II
Not provided
Description
The Mesoporous Silica Nanoparticle Platform Technology (MSNP) is composed of silicasomes (lipid-coated porous silica nanoparticle) , proton gradients, and a phospholipid bilayer (LB) coating. These components collectively function as a nanocarrier, enabling the delivery of multiple APIs to targeted site of action. These nanocarriers prolong the drug's circulatory half-life and deliver high doses at the site of action with reduced systemic toxicity compared to the free drug.
Technology highlight
1) Biocompatible 2) Cellular-level delivery of hydrophobic drugs 3) Nanocarriers encapsulate, covalently attach, and/or adsorb therapeutic agents to overcome drug solubility problems 4) Large surface area and porous interior to accommodate multiple API
Technology main components
The Mesoporous Silica Nanoparticle (MSNP) system comprises 'n' nanocarriers, each characterized by the following components: (i) API; (ii) a silicasome structure featuring a defined surface with a network of multiple pores; (iii) a phospholipid bilayer coating the silicasome surface; and (iv) a cargo-trapping agent (e.g., trimethylammonium salts, alpha- cyclodextrin sulfate, trim ethylammonium, beta-cyclodextrin phosphate, trimethyl ammonium citrate, and trimethylammonium acetate) and a targeting peptide (optional). These structural components are organized at the submicron scale.
Information on the raw materials sourcing, availability and anticipated price
Not provided
Delivery device(s)
No delivery device
APIs compatibility profile
API desired features
Water-insoluble molecules
Small molecules
MSNP-based drug delivery systems include a range of antifungal agents such as amphotericin B, anidulafungin, caspofungin, fluconazole, flucytosine, isavuconazole, itraconazole, micafungin, posaconazole, nocodazole, and voriconazole. Additionally, antiviral agents such as tenofovir disoproxil fumarate, antibiotics including ciprofloxacin and levofloxacin, and hydrophobic anticancer agents such as iriotecan, paclitaxel, ellipticine, camptothecin, acyclovir diphosphate, dimyristoylglycerol, doxorubicin, and chlorambucil can be included in this portfolio.
Additional solubility data
Not provided
Additional stability data
Not provided
API loading: Maximum drug quantity to be loaded
75-90 wt%
API co-administration
More than 4 different APIs : Not provided
LogP
Min: -1
Max: 7.1
Suitable for low and highly hydrophobic drugs
Scale-up and manufacturing prospects
Scale-up prospects
Not provided
Tentative equipment list for manufacturing
Not provided
Manufacturing
Manufacturing of the MSNP: 1. Synthesize mesoporous silica spheres using iron oxide nanocrystals or gold(III) chloride trihydrate and TEOS. 2. NPs are modified with a hydrophilic trihydroxylsilylpropyl methlphosphonate to prevent aggregation 3. Dissolve API in DMSO or an appropriate solvent. 4. Load the drug into mesoporous nanoparticles. 5. Redisperse drug-loaded nanoparticles in a hydrophobic solvent, or DMSO. 6. Sonicate, homogenize, and filter through a 0.44 µm syringe filter. 7. Mix with NaOH and H₂O; heat at 80 °C. For drugs with higher concentrations, reduce the heating temperature.
Specific analytical instrument required for characterization of formulation
1. Pore characterization is performed using X-ray Diffraction (XRD) 2. Nitrogen Adsorption desorption experiment - Brunauer-Emmett-Teller
Clinical trials
08103
Identifier
NCT00734682
Link
https://clinicaltrials.gov/study/NCT00734682
Phase
Phase I
Status
Completed
Sponsor
University of California, San Francisco
More details
This is a Phase I study of Nanoliposomal CPT-11 in patients with Recurrent high-grade gliomas. Patients must have a histologically proven intracranial malignant glioma, which includes glioblastoma multiforme (GBM), gliosarcoma (GS), anaplastic astrocytoma (AA), anaplastic oligodendroglioma (AO), anaplastic mixed oligoastrocytoma (AMO), or malignant astrocytoma NOS (not otherwise specified). Patients who are wild type or heterozygous for the UGT1A1\*28 gene will received Nanoliposomal CPT-11. The total anticipated accrual will be approximately 36 patients (depending upon the actual MTD). The investigators hypothesis is that this new formulation of CPT-11 will increase survival over that seen in historical controls who have recurrent gliomas because CPT-11 will be encapsulated in a liposome
Purpose
A Phase I Trial of Nanoliposomal CPT-11 (NL CPT-11) in Patients With Recurrent High-Grade Gliomas
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2008-08-01
Anticipated Date of Last Follow-up
2015-01-05
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2014-12-01
Actual Completion Date
2014-12-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Patients with histologically proven intracranial malignant glioma are eligible . -All patients must sign an informed consent * Patients must be \> 18 years old, and with a life expectancy \> 8 weeks. * Patients must have a Karnofsky performance status of \> 60. * Patients must have recovered from the toxic effects of prior therapy * Patients must have adequate bone marrow function (WBC \> 3,000/µl, ANC \> 1,500/mm3, platelet count of \> 100,000/mm3, and hemoglobin \> 10 gm/dl), adequate liver function (SGOT and bilirubin \< 2 times ULN), and adequate renal function (creatinine \< 1.5 mg/dL and/or creatinine clearance \> 60 cc/min) Patients must have shown radiographic evidence for tumor progression by MRI or CT scan.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
34
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
3G-18-1
Identifier
NCT03739801
Link
https://clinicaltrials.gov/study/NCT03739801
Phase
Phase I/II
Status
Withdrawn
Sponsor
University of Southern California
More details
This phase I/II trial studies the side effects and best dose of MM-398 and ramucirumab in treating patients with gastric cancer or gastroesophageal junction adenocarcinoma. MM-398 contains a chemotherapy drug called irinotecan, which in its active form interrupts cell reproduction. MM-398 builds irinotecan into a container called a liposome which may be able to release the medicine slowly over time to reduce side effects and increase its ability to kill tumor cells. Immunotherapy with monoclonal antibodies, such as ramucirumab, may help the body's immune system attack the cancer, and may interfere with the ability of tumor cells to grow and spread. Giving MM-398 and ramucirumab together may work better in treating patients with gastric cancer or gastroesophageal junction adenocarcinoma.
Purpose
MM-398 and Ramucirumab in Treating Patients With Gastric Cancer or Gastroesophageal Junction Adenocarcinoma
Interventions
Intervention 1
Intervention 2
Intervention 3
Intervention 4
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2020-04-06
Actual Start Date
Not provided
Anticipated Date of Last Follow-up
2020-03-25
Estimated Primary Completion Date
2022-04-06
Estimated Completion Date
2023-04-06
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * The patient has a histopathologically or cytologically confirmed diagnosis of gastric or gastroesophageal junction (GEJ) adenocarcinoma * The patient has metastatic disease or locally advanced and unresectable disease that is evaluable, by radiological imaging per Response Evaluation Criteria in Solid Tumors, Version 1.1 (RECIST 1.1). (MRI candidates must have measurable disease in liver. * The patient has documented disease progression or intolerance of chemotherapy during first-line platinum-based chemotherapy for metastatic disease, or during or within 6 months after the last dose of neoadjuvant or adjuvant therapy * Additional lines of therapy are permitted as long as patient had received a platinum and/or a fluoropyrimidine component.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
Not provided
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
PEP0208
Identifier
NCT00813163
Link
https://clinicaltrials.gov/study/NCT00813163
Phase
Phase II
Status
Completed
Sponsor
PharmaEngine
More details
The purpose of this study is to see the effect of PEP02 in the treatment of metastatic pancreatic cancer.
Purpose
Study of PEP02 as a Second Line Therapy for Metastatic Pancreatic Cancer
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2009-01-01
Anticipated Date of Last Follow-up
2019-08-27
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2010-12-01
Actual Completion Date
2012-07-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Histologically or cytologically confirmed adenocarcinoma of exocrine pancreas * Metastatic disease * Documented disease progression after treatment with 1 line of prior gemcitabine-based regimen * Karnofsky performance status equal or more than 70 Exclusion Criteria: * With active CNS metastases * With clinically significant gastrointestinal disorder (e.g., bleeding, inflammation, occlusion, or diarrhea \> grade 1) * Major surgery or radiotherapy within 4 weeks * Prior participation in any investigational drug study within 4 weeks * With prior irinotecan treatment
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
41
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
Excipients
Proprietary excipients used
No proprietary excipient used
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
No novel excipient or existing excipient used
Residual solvents used
No residual solvent used
Additional features
Other features of the technology
- Biodegradable
- Drug-eluting
- Non-removable
- Reservoir-type
- Requires stimuli from outside the body
Release properties
The release kinetics of MSNP are based on interactions between particles and cell membrane phospholipids during endocytosis, which can induce the release of encapsulated hydrophobic drugs. MSNP is designed to employ mechanical regulation of pore openings on the surface of NPs. Specifically, polymers, either adsorbed onto or covalently bonded to the MSNP surface, have been utilized as mechanized systems for controlled drug release.
Injectability
MSNP preparations can be formulated for intravenous, subcutaneous, and intramuscular routes of administration.
Safety
A Phase I clinical trial of mesoporous silica nanoparticle-encapsulated irinotecan (MSNP-IRI) was conducted, starting at a dose of 120 mg/m² with incremental increases of 60 mg/m², up to a maximum dose of 150 mg/m². Dose-limiting toxicities (DLTs) observed included diarrhea, dehydration, and fatigue. Notably, MSNP-IRI did not exhibit any unexpected toxicities when administered intravenously.
Stability
In Situ Stability: The mesoporous silica nanoparticles (MSNPs) exhibit excellent colloidal and circulatory stability in physiological fluids at pH 7.4, maintaining a monodisperse state to facilitate systemic biodistribution. Product Stability: While chronic shelf-life studies are yet to be undertaken, preliminary analyses indicate that the formulation remains stable for up to six months under cold storage conditions.
Storage conditions and cold-chain related features
At least 6 months is possible when stored at 4 ° C
Potential application(s)
Therapeutic area(s)
Use case(s)
Use of technology
Ease of administration
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
Frequency of administration
Every 3 weeks; Every 2 weeks
User acceptance
Not provided
Targeted user groups
Age Cohort- Adults
- Older Adults
- All
Pregnant individuals
Unspecified
Lactating individuals
Unspecified
Healthy individuals
Unspecified
Comment
Not provided
Potential associated API(s)
irinotecan
Class(es)
Topoisomerase 1 inhibitors
Development stage
Phase II
Clinical trial number(s)
NCT00813163
Foreseen/approved indication(s)
Gliomas and solid tumors
Foreseen user group
Adults who are < 18 years old with recurrent glioma
Foreseen duration between application(s)
Every 3 weeks
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
camptothecin
Class(es)
Topoisomerase - 1 inhibitors
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Pancreatic ductal Adenocarcinoma (PDAC)
Foreseen user group
Not provided
Foreseen duration between application(s)
Not provided
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
paclitaxel, taxanes
Class(es)
Taxane
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Pancreatic Duct Adenocarcinoma (PDAC)
Foreseen user group
Not provided
Foreseen duration between application(s)
Not provided
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Patent info
Description
Cationic polymer coated mesoporous silica nanoparticles and uses thereof
Brief description
A submicron structure having a silica body defining a plurality of pores is described. The submicron body may be spherical or non-spherical, and may include a cationic polymer or co-polymer on the surface of said silica body. The submicron structure may further include an oligonucleotide and be used to deliver the oligonucleotide to a cell. The submicron structure may further include a therapeutic agent and be used to deliver the therapeutic agent to a cell. An oligonucleotide and therapeutic agent may be used together. For example, when the oligonucleotide is an siRNA, the composition may be used to decrease cellular resistance to the therapeutic agent by decreasing translation of a resistance gene.
Representative patent
US10343903B2
Category
Formulation
Patent holder
The Regents of the University of California
Exclusivity
Not provided
Expiration date
July 13, 2031
Status
Active
Description
MESOPOROUS SILICA NANOPARTICLES FOR BIOMEDICAL APPLICATIONS
Brief description
A submicron structure includes a silica body defining a plurality of pores that are suitable to receive molecules therein , the silica body further defining an outer surface between pore openings of said plurality of pores ; and a plurality of anionic molecules attached to the outer surface of the silica body . The anionic molecules provide hydrophilicity to the submicron structure and are suitable to provide repulsion between other similar submicron structures, and the submicron structure has a maximum dimension less than one micron .
Representative patent
US10668024B2
Category
Formulation
Patent holder
The Regents of the University of California
Exclusivity
Not provided
Expiration date
December 8, 2028
Status
Expired - Fee Expired
Supporting material
Publications
<p><span style="color: rgb(33, 33, 33);">Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Mesoporous silica nanoparticles in biomedical applications. </span><em style="color: rgb(33, 33, 33);">Chem Soc Rev</em><span style="color: rgb(33, 33, 33);">. 2012;41(7):2590-2605. doi:</span><a href="10.1039/c1cs15246g" rel="noopener noreferrer" target="_blank" style="color: rgb(33, 33, 33);">10.1039/c1cs15246g</a></p>
This tutorial review provides an outlook on nanomaterials that are currently being used for theranostic purposes, with a special focus on mesoporous silica nanoparticle (MSNP) based materials. MSNPs with large surface area and pore volume can serve as efficient carriers for various therapeutic agents. The functionalization of MSNPs with molecular, supramolecular or polymer moieties, provides the material with great versatility while performing drug delivery tasks, which makes the delivery process highly controllable. This emerging area at the interface of chemistry and the life sciences offers a broad palette of opportunities for researchers with interests ranging from sol-gel science, the fabrication of nanomaterials, supramolecular chemistry, controllable drug delivery and targeted theranostics in biology and medicine.
<p><span style="color: rgb(33, 33, 33);">Lu J, Liong M, Sherman S, et al. Mesoporous Silica Nanoparticles for Cancer Therapy: Energy-Dependent Cellular Uptake and Delivery of Paclitaxel to Cancer Cells. </span><em style="color: rgb(33, 33, 33);">Nanobiotechnology</em><span style="color: rgb(33, 33, 33);">. 2007;3(2):89-95. doi:</span><a href="10.1007/s12030-008-9003-3" rel="noopener noreferrer" target="_blank" style="color: rgb(33, 33, 33);">10.1007/s12030-008-9003-3</a></p>
Biocompatible mesoporous silica nanoparticles, containing the fluorescence dye fluorescein isothiocyanate (FITC), provide a promising system to deliver hydrophobic anticancer drugs to cancer cells. In this study, we investigated the mechanism of uptake of fluorescent mesoporous silica nanoparticles (FMSN) by cancer cells. Incubation with FMSN at different temperatures showed that the uptake was higher at 37 degrees C than at 4 degrees C. Metabolic inhibitors impeded uptake of FMSN into cells. The inhibition of FMSN uptake by nocodazole treatment suggests that microtubule functions are required. We also report utilization of mesoporous silica nanoparticles to deliver a hydrophobic anticancer drug paclitaxel to PANC-1 cancer cells and to induce inhibition of proliferation. Mesoporous silica nanoparticles may provide a valuable vehicle to deliver hydrophobic anticancer drugs to human cancer cells.
Additional documents
No documents were uploaded
Useful links
There are no additional links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Illustrations
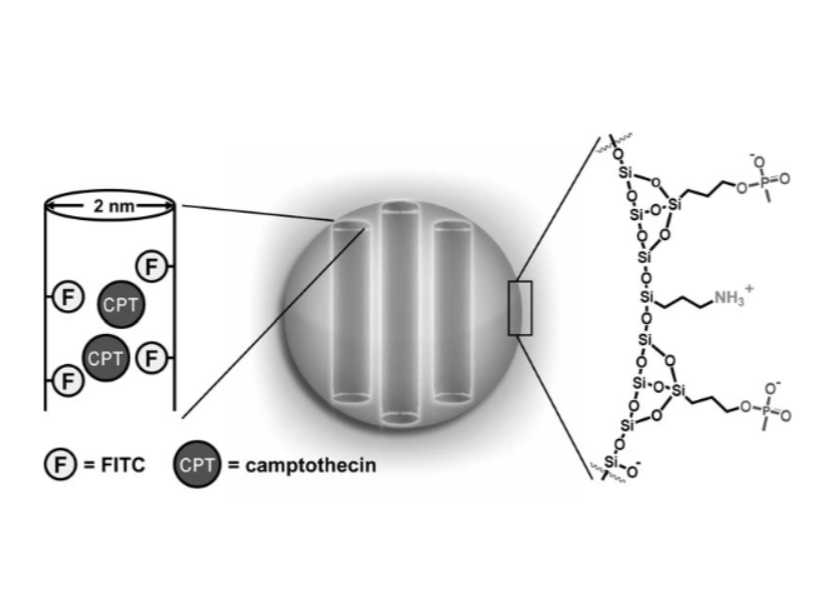
Schematic diagram of Camptothecin loaded nanoparticles
Lu, J., Liong, M., Zink, J. I., & Tamanoi, F. (2007). Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs. Small (Weinheim an der Bergstrasse, Germany), 3(8), 1341–13
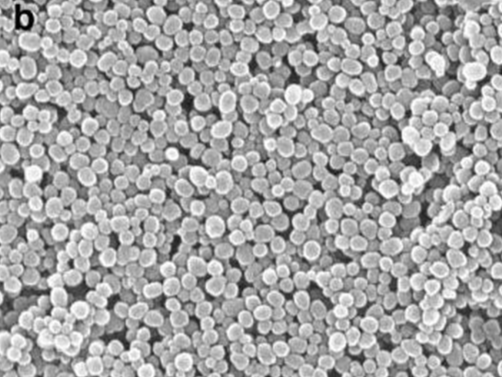
Scanning Electron Microscope of MSNP loaded with Paclitaxel
Lu J, Liong M, Sherman S, et al. Mesoporous Silica Nanoparticles for Cancer Therapy: Energy-Dependent Cellular Uptake and Delivery of Paclitaxel to Cancer Cells. Nanobiotechnology. 2007;3(2):89-95.

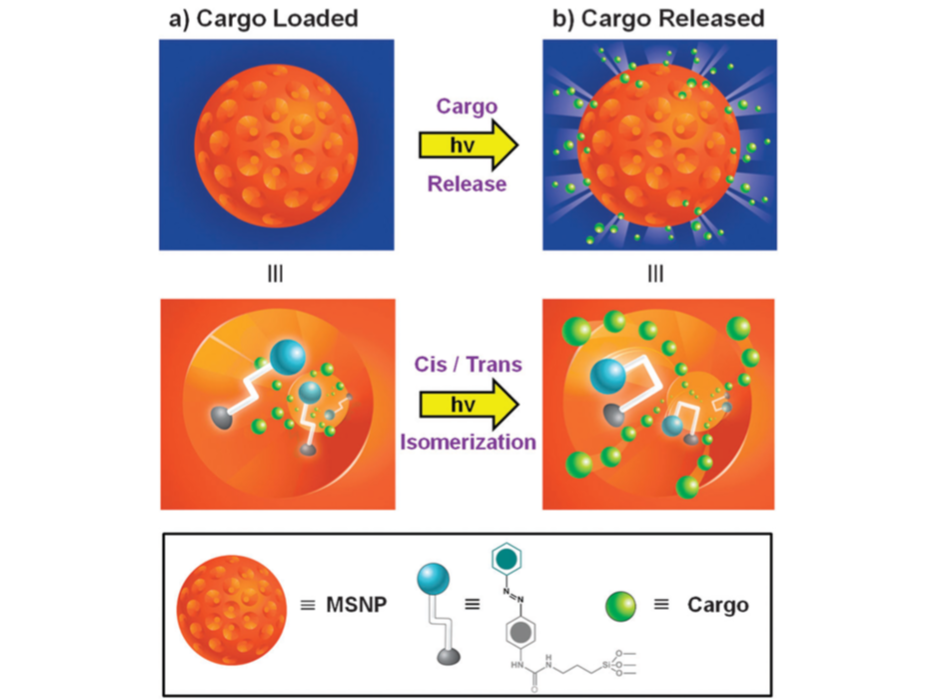
Mesoporous Nanoparticle Mechanism of Release of API
Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev. 2012;41(7):2590-2605. doi:10.1039/c1cs15246g
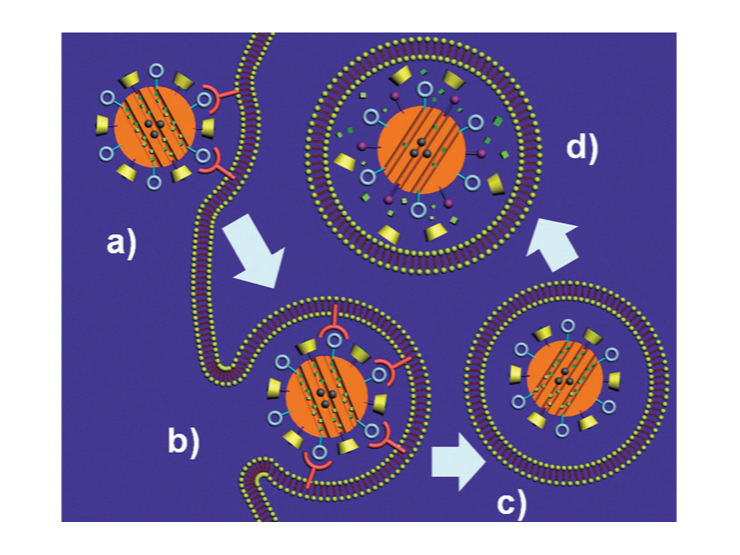
Endocytosis of MSNP
Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev. 2012;41(7):2590-2605. doi:10.1039/c1cs15246g