
|
Developed by 

|
Supported by 

|

Non-ARV/Non-Hormonal Dual-Purpose Monthly Vaginal Ring (Onering) (MATRIX-003)
Developer(s)

|
Oak Crest Institute Originator
https://www.oak-crest.org/
United States of America The Oak Crest Institute of Science was founded in 2001 in Monrovia, California, by Dr. Marc Baum. It began as an independent, non-profit research organization with a mission to conduct impactful scientific research while training the next generation of scientists. Over the years, it has grown into a hub for interdisciplinary research, focusing on drug delivery and infectious diseases. |
Sponsor(s)

|
U.S. Agency for International Development (USAID) https://www.usaid.gov/ |

|
U.S. President's Emergency Plan for AIDS Relief (PEPFAR) https://www.hiv.gov/federal-response/pepfar-global-aids/pepfar |
Partnerships

|
Weill Cornell Medicine https://weill.cornell.edu/ |

|
Wits Reproductive Health and HIV Institute, University of the Witwatersrand https://www.wits.ac.za/ |

|
Contraceptive Research and Development (CONRAD) https://www.conrad.org/ |

|
RTI International https://www.rti.org/ |
Technology information
Type of technology
Intra-vaginal ring
Administration route
Topical (Vaginal)
Development state and regulatory approval
Soluble adenyl cyclase (sAC) inhibitors
Pre-clinical
Not provided
Description
The dual-purpose vaginal ring is a silicone-based, doughnut-shaped vaginal ring designed for monthly contraception and prevention of diseases. It has an outer diameter of 55 mm and incorporates two drug delivery cassettes. One cassette contains a novel antiviral peptide, which is a protein fragment, while the other cassette delivers a hormone-free contraceptive agent—a soluble adenyl cyclase (sAC) inhibitor. Both active agents are released simultaneously at a controlled rate over a 28-day period.
Technology highlight
1. Sustained Release: Enables controlled and simultaneous release of two distinct drugs within the vaginal flora, ensuring localized and prolonged therapeutic efficacy. 2. Self-Administration: Designed for ease of use, allowing users to independently insert and remove the device. 3. Cost-Effectiveness: Low manufacturing costs make it an accessible option for widespread use. 4. Extended Shelf Life: Exhibits a longer shelf life compared to other long-acting contraceptive formulations, enhancing storage stability and usability.
Technology main components
The Onering is a silicone-based, doughnut-shaped vaginal ring featuring two distinct lobes. Each lobe consists of a reservoir support structure enclosed by a rate-controlling membrane and sealed with a reservoir end cap. (i) Lobe One contains a novel antiviral peptide, which is a protein fragment. (ii) Lobe Two contains a soluble adenyl cyclase (sAC) inhibitor.
Information on the raw materials sourcing, availability and anticipated price
Not provided
Delivery device(s)
No delivery device
APIs compatibility profile
API desired features
Small molecules
Soluble adenyl cyclase (sAC) inhibitors, including TDI-10229, LRE1, KH7, ADCY10, and TDI-11861, can be utilized in the dual-purpose design of the Onering/MATRIX-003. These inhibitors are selected for their slower dissociation rates and efficient local absorption within the vaginal environment. Currently, sAC inhibitors, whether as first- or second-line agents, have not yet received approval from any regulatory authority.
Proteins
Antiviral peptides, effective against human immunodeficiency virus (HIV), human papillomavirus (HPV), and herpes simplex virus (HSV), are targeted as a component of combination therapy in the Onering. These antivirals include (i) HIV: Enfuvirtide (T20), C34, TAT-derived peptides, VIRIP, Griffithsin; (ii) HPV: P18, L2-based peptides, HPV-16E7 peptides, RG1 epitope-derived peptides, Mimotopes; (iii) HSV: LL-37, TAT-CaM fusion peptides, BVD-21, G2 peptide, EP-100. Out of these peptides, Enfuvirtide (T20) is currently approved by USFDA (United States Food and Drug Adminstration).
Additional solubility data
Not provided
Additional stability data
Not provided
API loading: Maximum drug quantity to be loaded
50-75 wt%
API co-administration
2 different APIs : one API should be an antiviral peptide and other API should be a sAC inhibitor (an hormonal free contraceptive)
LogP
Not provided
Scale-up and manufacturing prospects
Scale-up prospects
Not provided
Tentative equipment list for manufacturing
Not provided
Manufacturing
Not provided
Specific analytical instrument required for characterization of formulation
Not provided
Clinical trials
MATRIX-003
Identifier
NCT06163274
Link
https://clinicaltrials.gov/study/NCT06163274
Phase
Pre-clinical
Status
Recruiting
Sponsor
University of Pittsburgh
More details
This research study is being conducted to find out how easy, comfortable, and safe intravaginal rings are for women to use. The two rings used in this study do not dispense any medications, are the same size, but differ in their flexibility and hardness. This study will enroll approximately 100 HIV-negative persons, aged18-45 years, and assigned female sex at birth from sites in the United States, South Africa, and Zimbabwe. Participants will be randomly assigned to use (self-insert) Ring A for 4 weeks and then Ring B for 4 weeks or Ring B first followed by Ring A. There will be a 1-3-week rest period between using the two different rings. The study involves answering questions, undergoing pelvic examinations, and collecting blood and vaginal fluid samples over a total of 7 in-person visit
Purpose
Trial to Assess Acceptability and Safety of Two Placebo Intravaginal Rings
Interventions
Intervention 1
Intervention 2
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2024-02-28
Anticipated Date of Last Follow-up
2024-03-21
Estimated Primary Completion Date
2024-12-01
Estimated Completion Date
2025-12-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
Genders
- Female
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
Yes
Comments about the studied populations
Inclusion Criteria: * Assigned female sex at birth. * Able and willing to provide written informed consent to be screened for and enrolled in MATRIX-003 in one of the study languages. * Able and willing to provide adequate contact/locator information. * Able and willing to comply with all protocol requirements, including: * Abstaining from other intravaginal products or practices for the duration of the study. * Abstaining from penetrative vaginal intercourse (i.e., oral-, digital-, penile-penetration) for the first 14 days of each product use period. * Refraining from participation in other research studies involving drugs, medical devices, vaginal products, or vaccines starting 2 weeks before the Screening Visit and for the duration of the study, or in observational or qualitativ
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
100
Allocation
Randomized
Intervention model
Cross-over assignment
Intervention model description
Not provided
Masking
Single blind masking
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
PrEP
Key results
Excipients
Proprietary excipients used
No proprietary excipient used
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
No novel excipient or existing excipient used
Residual solvents used
No residual solvent used
Additional features
Other features of the technology
- Drug-eluting
- Monolithic
- Removable
- Single-use
- Molded
- Reservoir-type
- At least 1 year shelf life
Release properties
Not provided
Injectability
Not applicable
Safety
The safety outcomes of the Early Phase 1 trial (MATRIX-003) have not yet been disclosed, as the study only commenced in the first quarter of 2024.
Stability
The shelf life of the formulation is longer than most of the long acting formulation i.e., 2 years.
Storage conditions and cold-chain related features
Store at a temperature range of 15°C to 30°C (59°F to 86°F).
Potential application(s)
Therapeutic area(s)
Use case(s)
Use of technology
Ease of administration
- Self-administered
Frequency of administration
Monthly
User acceptance
Not provided
Targeted user groups
Age Cohort- Adults
- Older Adults
- Female
- Cisgender female
- Transgender female
Pregnant individuals
Unspecified
Lactating individuals
Unspecified
Healthy individuals
Unspecified
Comment
Not provided
Potential associated API(s)
Other antivirals
Class(es)
Antiviral Peptides
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
HIV, HSV and HPV
Foreseen user group
Not provided
Foreseen duration between application(s)
Once monthly
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Soluble adenyl cyclase (sAC) inhibitors
Class(es)
Hormone Free Contraceptive
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Contraception
Foreseen user group
Not provided
Foreseen duration between application(s)
Once monthly
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Patent info
There are either no relevant patents or these were not yet submitted to LAPaL
Supporting material
Publications
<p><span style="color: rgb(33, 33, 33);">Miller, M., Rossetti, T., Ferreira, J., Ghanem, L., Balbach, M., Kaur, N., Levin, L. R., Buck, J., Kehr, M., Coquille, S., van den Heuvel, J., Steegborn, C., Fushimi, M., Finkin-Groner, E., Myers, R. W., Kargman, S., Liverton, N. J., Huggins, D. J., & Meinke, P. T. (2022). Design, Synthesis, and Pharmacological Evaluation of Second-Generation Soluble Adenylyl Cyclase (sAC, ADCY10) Inhibitors with Slow Dissociation Rates. </span><em style="color: rgb(33, 33, 33);">Journal of medicinal chemistry</em><span style="color: rgb(33, 33, 33);">, </span><em style="color: rgb(33, 33, 33);">65</em><span style="color: rgb(33, 33, 33);">(22), 15208–15226. </span><a href="https://doi.org/10.1021/acs.jmedchem.2c01133" rel="noopener noreferrer" target="_blank" style="color: rgb(33, 33, 33);">https://doi.org/10.1021/acs.jmedchem.2c01133</a></p>
Soluble adenylyl cyclase (sAC: ADCY10) is an enzyme involved in intracellular signaling. Inhibition of sAC has potential therapeutic utility in a number of areas. For example, sAC is integral to successful male fertility: sAC activation is required for sperm motility and ability to undergo the acrosome reaction, two processes central to oocyte fertilization. Pharmacologic evaluation of existing sAC inhibitors for utility as on-demand, nonhormonal male contraceptives suggested that both high intrinsic potency, fast on and slow dissociation rates are essential design elements for successful male contraceptive applications. During the course of the medicinal chemistry campaign described here, we identified sAC inhibitors that fulfill these criteria and are suitable for in vivo evaluation of diverse sAC pharmacology.
<p><span style="color: rgb(33, 33, 33);">Palanee-Phillips, T., Baum, M. M., Moss, J. A., Clark, M. R., Nuttall, J., & Romano, J. W. (2022). Drug-releasing vaginal rings for HIV/STI and pregnancy prevention: a review of recent advances and clinical applications. </span><em style="color: rgb(33, 33, 33);">Expert opinion on drug delivery</em><span style="color: rgb(33, 33, 33);">, </span><em style="color: rgb(33, 33, 33);">19</em><span style="color: rgb(33, 33, 33);">(1), 47–58. </span><a href="https://doi.org/10.1080/17425247.2022.2020242" rel="noopener noreferrer" target="_blank" style="color: rgb(33, 33, 33);">https://doi.org/10.1080/17425247.2022.2020242</a></p>
Introduction: Adolescent girls and young women (AGYW), as well as pre- and post-menopausal women globally would benefit from expanded choice to address their sexual and reproductive health (SRH) needs related to Human Immunodeficiency Virus (HIV), sexually transmitted infections (STIs) and pregnancy prevention. Lack of adequate preventative vaccines for HIV/STIs reinforces public health prioritization for options women may use to mitigate risk for infectious disease and unplanned pregnancy. Drug releasing intravaginal rings (IVRs) represent one such technology that has garnered attention based on the modality's success as a pre-exposure prophylaxis (PrEP) delivery option in HIV risk reduction.
Areas covered: This article provides a synopsis of three IVR technologies in active clinical development for prevention of HIV, STI, and unintended pregnancy demonstrating advancements in terms of compatibility with a wide range of drug types with a focus on dapivirine-based silicone rings (International Partnership for Microbicides (IPM), tenofovir-based polyurethane rings (Conrad), and pod-based rings (Oak Crest Institute of Science)).
Expert opinion: The goals of IVR research are to reduce burdens of HIV/STIs and unplanned pregnancies. Through the evolution of IVR technologies, the potential exists to trigger integration of health-care services through formulation of products with multiple indications
<p><span style="color: rgb(33, 33, 33);">Ridgeway, K., Montgomery, E. T., Smith, K., Torjesen, K., van der Straten, A., Achilles, S. L., & Griffin, J. B. (2022). Vaginal ring acceptability: A systematic review and meta-analysis of vaginal ring experiences from around the world. </span><em style="color: rgb(33, 33, 33);">Contraception</em><span style="color: rgb(33, 33, 33);">, </span><em style="color: rgb(33, 33, 33);">106</em><span style="color: rgb(33, 33, 33);">, 16–33. </span><a href="https://doi.org/10.1016/j.contraception.2021.10.001" rel="noopener noreferrer" target="_blank" style="color: rgb(33, 33, 33);">https://doi.org/10.1016/j.contraception.2021.10.001</a></p>
Objective: The vaginal ring (ring) is a female-initiated, long-acting drug delivery system for different indications, including HIV prevention. Our aim was to provide evidence for acceptability of the vaginal ring across indications to support dapivirine and multipurpose prevention technology ring introduction and roll out.
Study design: This systematic review and meta-analysis followed PRISMA guidelines. We searched PubMed, Web of Science, Embase, and grey literature for publications reporting favorable ring acceptability and secondary outcomes involving actual ring use (comfort, ease of ring use, ring comfort during sex, expulsions, and vaginal symptoms) or hypothetical acceptability for any indication published January 1, 1970-June 15, 2021. We estimated random-effects pooled prevalence, assessing between-study variation using meta-regression.
Results: Of 2,234 records, we included 123 studies with 40,434 actual and hypothetical ring users. The primary outcome assessment included 50 studies with 60 ring subgroups totaling 19,271 ring users. The favorable acceptability pooled prevalence was 85.6% (95%CI 81.3, 89.0), while hypothetical acceptability among non-ring users was 27.6% (95%CI 17.5, 40.5). In meta-regression, acceptability was higher in menopause (95.4%; 95%CI 88.4, 98.2) compared to contraceptive rings (83.7%; 95%CI 75.6, 89.5). Acceptability was lower in pharmacokinetic studies (50%; 95%CI 22.1, 77.9) compared to RCTs (89.5%; 95%CI 85.8.92.4) and in studies assessing acceptability at ≥12 months (78.5%; 95%CI 66.5, 87.1) versus studies assessing acceptability at <3 months (91.9%; 95%CI 83.7, 96.1). European (90.6%; 95%CI 83.9, 94.7), Asian (97.1%; 95%CI 92.0, 99.0), and multi-region studies (93.5%; 95%CI 84.6, 97.4) reported more favorable acceptability compared to African studies (59.4%; 95%CI 38.3, 77.5). Secondary outcomes were similarly favorable, including ring comfort (92.9%; 95%CI 89.2, 95.4), ease of use (90.9%; 95%CI 86.5, 94.0), and comfort during sex (82.7%; 95%CI 76.4, 87.6). Limitations include inconsistent outcome definitions and unmeasured factors affecting acceptability.
Conclusions: Women who used vaginal rings reported they were acceptable across indications geographic regions and indications. Policy makers should consider the ring as an important option for pregnancy and HIV prevention drug development.
Implications: This review found favorable acceptability among vaginal ring users across indications and geographic areas, in contrast to low hypothetical acceptability among non-users. Vaginal rings are an important drug delivery system for pregnancy and HIV preventions, and scale-up should plan to address initial hesitancy among new users.
<p><span style="color: rgb(33, 33, 33);">Minnis, A. M., Etima, J., Musara, P., Browne, E. N., Mutero, P., Kemigisha, D., Mgodi, N. M., Nakabiito, C., Shapley-Quinn, M. K., Stoner, M. C. D., Hartmann, M., Macagna, N., Piper, J., & van der Straten, A. (2022). Couples' Preferences for "2 in 1" Multipurpose Prevention Technologies to Prevent Both HIV and Pregnancy: Results of a Discrete Choice Experiment in Uganda and Zimbabwe. </span><em style="color: rgb(33, 33, 33);">AIDS and behavior</em><span style="color: rgb(33, 33, 33);">, </span><em style="color: rgb(33, 33, 33);">26</em><span style="color: rgb(33, 33, 33);">(12), 3848–3861. </span><a href="https://doi.org/10.1007/s10461-022-03713-6" rel="noopener noreferrer" target="_blank" style="color: rgb(33, 33, 33);">https://doi.org/10.1007/s10461-022-03713-6</a></p>
End-user input early in biomedical product development may optimize design to support high uptake and adherence. We interviewed 400 couples (800 total participants) in Uganda and Zimbabwe to assess their preferences for multipurpose prevention technologies (MPTs) for HIV and pregnancy prevention. Using a discrete choice experiment, couples made a series of choices between hypothetical MPTs, including oral tablets and vaginal rings, inserts, and films and completed an interviewer-administered questionnaire assessing sociodemographic and behavioral measures. Most couples preferred presented MPTs over male condoms. Couples' MPT choices in both countries were influenced most by the combination of product form and dosing frequency, with monthly dosing preferred over daily. Analysis highlighted differences by country as to which side effects were most important: Ugandan couples placed greater importance on effects on the vaginal environment during sex, whereas Zimbabwean couples placed more importance on changes to menstruation and other side effects (headache, cramps). Couples' preferences signaled an openness to new product forms and more frequent dosing if preferred characteristics of other attributes were achieved.
Useful links
There are no additional links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Illustrations
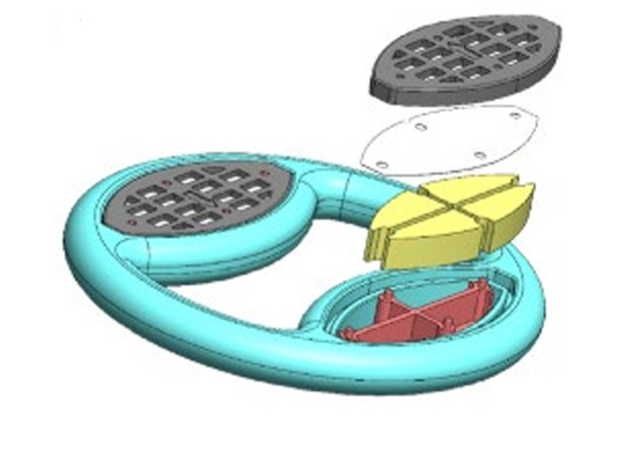
Drug-loaded Lobes of Flexible Elastomeric Vaginal Ring with multiple components for drug storage and a release member
Matrix for Prevention. (2023). MATRIX-003 Protocol Version 1.0. https://www.matrix4prevention.org/sites/default/files/2023-10/MATRIX-003_Protocol%20Version%201.0_Final_06.29.23.pdf

Non-ARV/Non-Hormonal Dual-Purpose Monthly Vaginal Ring
Matrix for Prevention. (2024, March 20). Ring and MATRIX-003 QA. https://www.matrix4prevention.org/sites/default/files/2024-03/Ring%20and%20MATRIX-003%20QA_%2020March2024.pdf