
|
Developed by 

|
Supported by 

|

NanoPortal™
Based on public informationDeveloper(s)

|
Vivani Medical Originator
https://vivani.com/
United States Vivani Medical, Inc., headquartered in Alameda, California, develops biopharmaceutical implants leveraging their proprietary NanoPortal™ platform. The company focuses on creating long-term drug delivery solutions to address chronic diseases such as type 2 diabetes and chronic weight management. Vivani was formed from the merger of Nano Precision Medical, Inc. and Second Sight Medical Products, Inc |
Sponsor(s)
|
No sponsor indicated |
Partnerships

|
NanoPrecision Medical https://www.linkedin.com/company/nanoprecision-medical/ |
Technology information
Type of technology
Titanium implant
Administration route
Subcutaneous, Intraocular
Development state and regulatory approval
Exenatide
Phase I
Not provided
Description
The NanoPortal implant device technology are customizable drug delivery system according to the desired drug release rate, implant duration, and various other factors specific to the target product profile. NanoPortal holds significant potential to improve tolerability by addressing common issues associated with the API and its variable drug release patterns. Smaller nanotube pore size and fewer exposed nanotubes produces slower drug release rates.
Technology highlight
• Space-Efficient Design • No pumps or electronic devices • Subdermal Administration • Incorporation of different concentrations of API • Vertical nanotubes (40 micrometers in length) attached to titanium substrate • The content of the tubes are customizable depending on the desired delivery rate of the API • Pore is only slightly larger than the API molecule, you can achieve a near constant steady rate of medication deliver
Technology main components
The NanoPortal implant typically consists of • A housing (Capsule) • Titania (TiO2) nanotube membrane • Obturator • Pressure reducer • Connector for transport of fluid • Reservoir for biocompatible fluid
Information on the raw materials sourcing, availability and anticipated price
Not provided
Delivery device(s)
NanoPortal Implantable drug delivery system: Titanium-based subcutaneous implant with nanopore membrane for drug delivery
APIs compatibility profile
API desired features
Water-soluble molecules
Water-insoluble molecules
Small molecules
Therapeutic agents of type 2 diabetes, high blood pressure, heart disease, stroke, joint problems, liver disease, gallstones, some types of cancer, and sleep & pulmonary diseases are targeted for NanoPortal Implant drug delivery system.
Proteins
NanoPortal has the potential to deliver large hydrophilic molecules, such as peptides and proteins, potentially enabling a broader range of therapeutic applications.
Additional solubility data
Not provided
Additional stability data
Not provided
API loading: Maximum drug quantity to be loaded
Not provided
API co-administration
Not provided
LogP
Not provided
Scale-up and manufacturing prospects
Scale-up prospects
Not provided
Tentative equipment list for manufacturing
• Plasma treatment system • Uv-ozone treatment unit • Chemical activation bath • Dip-coating machine • Spin-coating device • Spray-coating equipment • Layer-by-layer assembly setup • Thermal curing ovens • Chemical curing stations • Annealing furnaces • Controlled atmosphere chambers
Manufacturing
ISO Class 5 to ISO Class 8 with HEPA filters Process of manufacturing includes: • Preparation of Nanoporous Substrate: Create nanopores in polycarbonate using ion track etching/ anodization/ phase inversion • Surface Treatment and Coating Preparation: Enhance adhesion with plasma treatment or chemical activation; prepare the coating solution with desired materials and additives • Application and Curing of Coating: Apply the coating via dip-coating or spray-coating, then dry and cure using thermal treatment, UV irradiation, or chemical curing • Post-Treatment and Characterization using SEM.
Specific analytical instrument required for characterization of formulation
• Scanning Electron Microscopy (SEM) • Atomic Force Microscopy (AFM) • Fourier Transform Infrared Spectroscopy (FTIR) • Raman spectroscopy • Differential Scanning Calorimetry (DSC) • Thermogravimetric Analysis (TGA) • Tensile testing • Nanoindentation • Gas Adsorption (BET Analysis) • Mercury Intrusion Porosimetry • High-Performance Liquid Chromatography (HPLC) • Mass Spectrometry (MS) • X-ray Photoelectron Spectroscopy (XPS)
Clinical trials
LIBERATE-1
Identifier
NCT05670379
Link
https://clinicaltrials.gov/study/NCT05670379
Phase
Phase I
Status
Not yet recruiting
Sponsor
Vivani Medical, Inc
More details
The purpose of this study is to evaluate the safety, tolerability and drug levels of an exenatide implant (NPM-119) for the treatment of type 2 diabetes
Purpose
Assessment of Safety, Tolerability and Drug Levels of NPM-119 in Participants With Type 2 Diabetes
Interventions
Intervention 1
Intervention 2
Countries
Not provided
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2024-03-01
Actual Start Date
Not provided
Anticipated Date of Last Follow-up
2023-11-27
Estimated Primary Completion Date
2025-01-01
Estimated Completion Date
2025-01-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Type 2 diabetes * BMI up to 40 kg/m\^2 * Estimated glomerular filtration rate (eGFR) \>60 mL/min/1.73 m\^2 * HbA1c \<8.5 * Treated with a stable regimen of a GLP-1receptor agonist other than exenatide-containing drugs for a minimum of 3 months Exclusion Criteria: * Has a clinically significant medical condition that could potentially affect study participation and/or personal well-being * History of, or currently has, acute or chronic pancreatitis or has triglyceride concentrations ≥500 mg/dL * Has medullary thyroid carcinoma (MTC) or multiple endocrine neoplasia (MEN II) or a family history of MTC or MEN II * Current or past exposure to exenatide * Sulfonylurea (SU) use within the prior 3 months * Alpha-glucosidase inhibitor, meglitinide, nateglinide.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
16
Allocation
Randomized
Intervention model
Parallel Assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
Excipients
Proprietary excipients used
No proprietary excipient used
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
No novel excipient or existing excipient used
Residual solvents used
No residual solvent used
Additional features
Other features of the technology
- Drug-eluting
- Removable
- Single-use
- Reservoir-type
Release properties
Minimally fluctuating drug release profile were observed in pre-clinical studies.
Injectability
Insertion using smaller 11-gauge needle
Safety
Phase I (LIBERATE-1) safety and efficacy studies of NPM-115 are ongoing.
Stability
Not provided
Storage conditions and cold-chain related features
Not provided
Potential application(s)
Therapeutic area(s)
Use case(s)
Not provided
Use of technology
Ease of administration
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
Frequency of administration
Weekly, Monthly, Every 12 weeks
User acceptance
Not provided
Targeted user groups
Age Cohort- Adults
- Older Adults
- All
Pregnant individuals
Unspecified
Lactating individuals
Unspecified
Healthy individuals
Unspecified
Comment
Not provided
Potential associated API(s)
Exenatide
Class(es)
GLP-1 agonist
Development stage
Phase I
Clinical trial number(s)
NCT05670379
Foreseen/approved indication(s)
Obesity, Type II Diabetes Mellitus, Feline Pre-Diabetes & Diabetes
Foreseen user group
Not provided
Foreseen duration between application(s)
Once every 12 weeks
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Semaglutide
Class(es)
GLP-1 Analogues
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Obesity
Foreseen user group
Not provided
Foreseen duration between application(s)
Not provided
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Patent info
Description
Implant Delivery System with Hydration Promotor Capability
Brief description
The application pertains to apparatuses, means and methods to promote uptake of biocompatible fluids into a reservoir of an implantable drug delivery system though a porous membrane. Examples of the application promote fluid uptake by creating a pressure differential between the reservoir of the drug delivery device and the biocompatible fluid outside the device.
Representative patent
WO2018067535
Category
Medical Device
Patent holder
Nano Precision Medical, Inc.
Exclusivity
Not provided
Expiration date
October 7, 2037
Status
Not provided
Description
Apparatus and Method for Promoting Fluid Uptake into an Implant
Brief description
The invention pertains to apparatuses, means and methods to promote uptake of fluids into a reservoir of an implantable drug delivery system though a porous membrane. Embodiments of the invention promote fluid uptake by creating a pressure differential between the reservoir of the drug delivery device and the environment of the device after implantation, for instance a subcutaneous pocket.
Representative patent
WO2016123027
Category
Medical Device
Patent holder
Nano Precision Medical, Inc.
Exclusivity
Not provided
Expiration date
January 26, 2036
Status
Not provided
Description
Implant Device for Drug Delivery
Brief description
The present invention provides a method for controlling the internal diameter of nanopores to afford nanopore membranes with a zero-order rate of release of a therapeutic agent.
Representative patent
WO2015112811A1
Category
Medical Device
Patent holder
Nano Precision Medical, Inc.
Exclusivity
Not provided
Expiration date
January 23, 2035
Status
Not provided
Supporting material
Publications
There are no publication
Additional documents
Useful links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Illustrations

NanoPortal Implant
NanoPortal (2024) Vivani. Available at: https://vivani.com/ (Accessed: 09 July 2024).
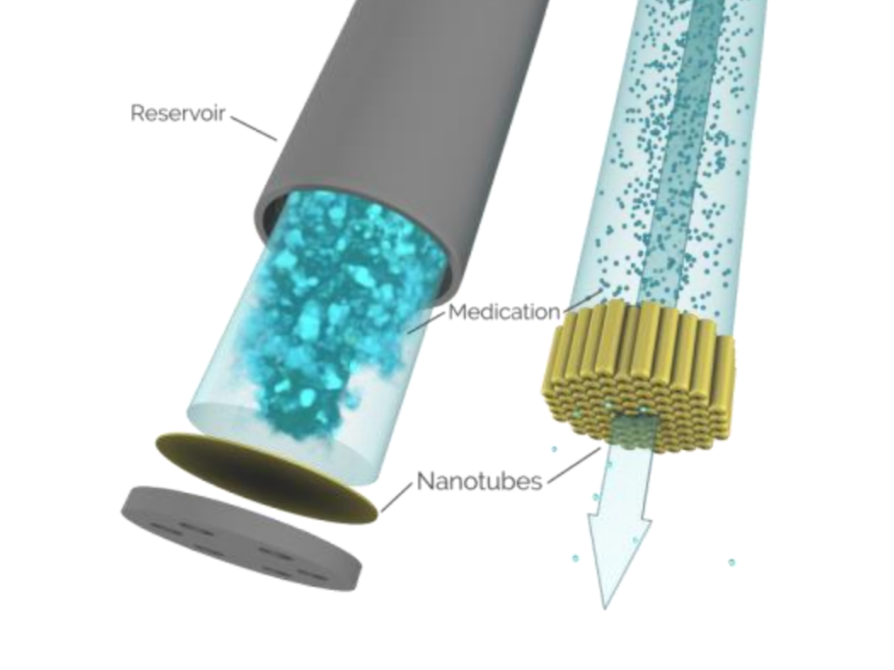
Graphical illustration of different parts of the NanoPortal
Vivani Medical. (2024, May 13). Vivani investor presentation May 2024. Retrieved from https://d1io3yog0oux5.cloudfront.net/_2ec16443bfa8ea8ed92ef0a0a7e08196/vivanimedical/db/2227/20832/pdf.
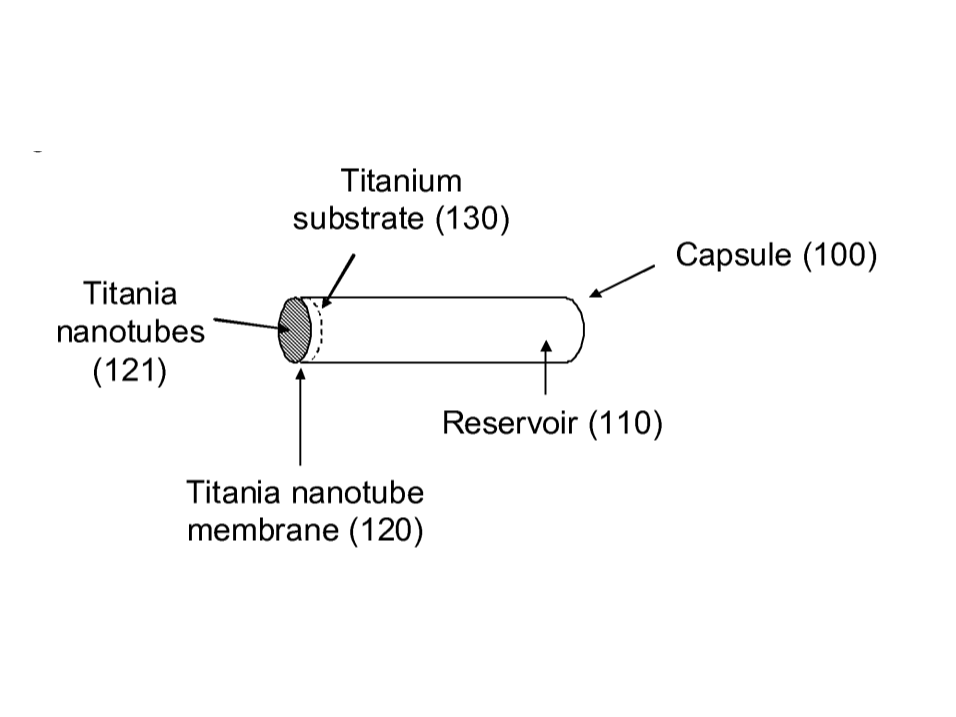
Different parts of the NanoPortal
Young, P., & Borde, B. (2015). System and method for facilitating development of a cellular therapy (WO2015112811A1). World Intellectual Property Organization. Retrieved from https://patents.google.co