
|
Developed by 

|
Supported by 

|
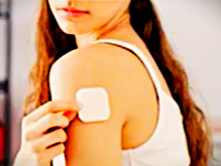
STARSILON Transdermal Patch
Based on public informationDeveloper(s)

|
Starton Therapeutics, Inc. Originator
https://www.startontx.com
United States Starton Therapeutics, a clinical-stage biotechnology enterprise established in 2017, is dedicated to advancing cancer treatment paradigms. The company's core focus is the development of innovative continuous delivery systems to augment existing cancer therapies. This technological approach aims to provide uninterrupted therapeutic exposure for patients with cancer. |
Sponsor(s)
|
No sponsor indicated |
Partnerships

|
Haisco Pharmaceuticals, Inc. https://en.haisco.com |
|
Healthwell Acquisition Corp. https://healthwellspac.com/ |
Technology information
Type of technology
Polymer-based particles, Transdermal patch
Administration route
Subcutaneous, Transdermal
Development state and regulatory approval
Olanzapine
Phase II
US Food and Drug Administration (FDA) has cleared an investigational new drug (IND) application for STAR-OLZ in Chemotherapy in 2022.
Description
The STARSILON long-acting transdermal patch is a self-administered dosage form that disperses the drug locally in a controlled fashion. This transdermal patch has the potential to modulate pharmacokinetic parameters, including reduced area under the curve (AUC), peak concentration (Cmax), and overall drug exposure. This approach presents opportunities for enhanced therapeutic efficacy and tolerability, as well as the exploration of novel indications or superior outcomes within established treatment areas.
Technology highlight
• Self-administered transdermal formulation and injectable options that provide controlled drug release for at least five days • Potential to have a lower incidence of dose-limiting side effects • Elimination of subtherapeutic drug levels due to short half-life APIs
Technology main components
• Pressure Sensitive Adhesive (Eg: Styrene - Butadiene - Styrene) • Solubilization Agent / Crystallization Inhibitor (Eg: uncrosslinked polyvinylpyrrolidone ( PVP )) • Thickener (Eg: cassia tora , collagen , gelatin , gellum gum , guar gum , pectin , potassium , or sodium carageenan , tragacanth , xantham , gum copal, chitosan , resin semisynthetic polymers and its derivatives: methylcellulose , ethyl cellulose , carboxymethyl cellulose , hydroxylpropyl cellulose (Klucel HF)) • Skin Permeation Enhancer (Eg: oleic acid , oleyl alcohol ) • Skin Modifiers (Eg: butylated hydroxytoluene; butylated hydroxyanisole) • Polar Aprotic Solvent (Eg: n - methyl - 2 - pyrrolidone (NMP)) • Backing layer (Eg: Scotchpak® 1012) • Release Liner (Eg: Bio - Release® liner) • Penetration enhancers
Information on the raw materials sourcing, availability and anticipated price
Raw materials for formulation include polyvinylpyrrolidone (PVP) sourced from BASF Pharma, hydroxypropyl cellulose (Klucel HF) from Ashland, carbomer (Carbopol) from Lubrizol Corporation, colloidal silicon dioxide (AEROSIL) and acrylic polymers (Eudragit) from Evonik Corporation, and polyoxyethylene glycol ether (Brij) from B.J. Bridge. The projected cost of the final product remains undisclosed at this time.
Delivery device(s)
Not provided
APIs compatibility profile
API desired features
Water-soluble molecules
Water-insoluble molecules
Small molecules
Immunomodulatory agents such as thalodomide, lenalidomide, pomalidomide, iberdomide in combination with dexamethasone, Dronabinol, apremilast analogs , apremilast derivatives , apremilast metabolites , and combinations thereof are targeted for this dosage form.
Additional solubility data
Not provided
Additional stability data
Not provided
API loading: Maximum drug quantity to be loaded
Not provided
API co-administration
2 different APIs : Not provided
LogP
Not provided
Scale-up and manufacturing prospects
Scale-up prospects
Not provided
Tentative equipment list for manufacturing
• Mixing Apparatus • Laminating Machine • Drying oven • Die-Cutting Machine • Solvent Evaporation System
Manufacturing
ISO Class 7 or 8 with HEPA filters. Manufacturing process includes: • Hot melt extrusion process • Pretreatment Composition • Blend Preparation • Coating
Specific analytical instrument required for characterization of formulation
• HPLC • Gas Chromatographly (GC) • Differential Scanning Calorimetry (DSC) • Fourier-Transform Infrared Spectroscopy (FTIR) • UV-Visible Spectroscopy • Scanning Electron Microscopy • Tensile Testing Equipment
Clinical trials
STAR LLD MM 023
Identifier
NCT06087653
Link
https://clinicaltrials.gov/study/NCT06087653
Phase
Phase I/II
Status
Recruiting
Sponsor
Starton Therapeutics, Inc
More details
Primary Objective • Assess the safety and tolerability of low-dose lenalidomide administered by continuous subcutaneous (SC) infusion (STAR-LLD) in combination with dexamethasone and a proteasome inhibitor (PI). Secondary Objectives * To assess the immunologic activity of natural killer (NK) cells and T cells for innate and humoral immunity. * To establish the pharmacokinetic (PK) profile of STAR-LLD at a defined infusion rate targeting steady-state blood concentrations. * To determine pharmacodynamic (PD) changes with STAR-LLD in a panel of biomarkers associated with clinical response to lenalidomide. * Evaluate changes in efficacy indicators including objective response rate (ORR), progression-free survival (PFS), and duration of response (DOR).
Purpose
Safety, Efficacy, and Pharmacokinetics of Continuous Subcutaneous Lenalidomide in Multiple Myeloma (MM)
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2023-10-02
Anticipated Date of Last Follow-up
2023-10-11
Estimated Primary Completion Date
2024-07-01
Estimated Completion Date
2024-09-01
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: 1. Male or female ≥18 years at the time of informed consent. 2. Autologous stem cell transplant (ASCT) ineligible. 3. SARS -CoV2 virus (COVID)-19 negative. 4. A prior diagnosis of MM as defined by International Myeloma Working Group (IMWG) criteria (Appendix 7). 5. Documented measurable disease following first line therapy defined as: * Serum monoclonal protein ≥1.0 g/dL by protein electrophoresis. * ≥200 mg/24 hours of monoclonal protein in the urine on 24-hour electrophoresis. * Serum free light chain (SFLC) ≥10 mg/dL AND abnormal serum kappa to lambda free light chain (FLC) ratio. 6. Intended to be treated in 2nd line or greater with lenalidomide, dexamethasone, and a PI.
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
6
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
Excipients
Proprietary excipients used
No proprietary excipient used
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
No novel excipient or existing excipient used
Residual solvents used
No residual solvent used
Additional features
Other features of the technology
- Drug-eluting
- Monolithic
- Removable
- Reservoir-type
- Other(s)
Ambulatory Subcutaneous pump
Release properties
• The release rate of the API from the transdermal patch is influenced by multiple factors, including drug solubility within the matrix, adhesive type, and the incorporation of permeation enhancers. • Preclinical pharmacokinetic studies comparing lenalidomide delivered transdermally to oral administration demonstrated more consistent drug plasma concentration profiles. • This suggests that transdermal delivery may bypass hepatic first-pass metabolism, potentially enhancing the drug's bioavailability.
Injectability
Not applicable
Safety
Interim results from the Phase 1b STAR-LLD trial (2024) demonstrated no hematologic toxicities exceeding Grade 1 after up to three treatment cycles. Additionally, no drug-related non-hematologic toxicities beyond Grade 1 were observed, with only a single instance of Grade 1 dermatologic toxicity reported across four cumulative cycles.
Stability
STARSILON transdermal patches offer a versatile drug delivery system, capable of providing sustained release from 24 hours up to 15 days. Stability and shelf life of the patch can be optimized through various pharmaceutical strategies, including co-crystal formation, drug coating, and the incorporation of inert protective agents.
Storage conditions and cold-chain related features
Not provided
Potential application(s)
Therapeutic area(s)
Use case(s)
Use of technology
Ease of administration
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
- Self-administered
Frequency of administration
Every 5 days
User acceptance
Not provided
Targeted user groups
Age Cohort- Adults
- Older Adults
- All
Pregnant individuals
Unspecified
Lactating individuals
Unspecified
Healthy individuals
Unspecified
Comment
Not provided
Potential associated API(s)
Olanzapine
Class(es)
Antipsychotic
Development stage
Phase II
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Chemotherapy-induced nausea and vomiting (CINV)
Foreseen user group
Not provided
Foreseen duration between application(s)
Once every 5 days
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
US Food and Drug Administration (FDA) has cleared an investigational new drug (IND) application for STAR-OLZ in Chemotherapy in 2022.
lenalidomide
Class(es)
CRL4CRBN E3 ubiquitin ligase modulator
Development stage
Phase I
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Multiple Myeloma and Chronic Lymphocytic Leukemia
Foreseen user group
Not provided
Foreseen duration between application(s)
Not provided
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
A comprehensive data summary for the U.S. Food and Drug Administration (FDA) will be compiled by the company. Additionally, phase II clinical trials are scheduled to commence in 2025.
dronabinol
Class(es)
Antiemetics
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Chemotherapy induced nausea and vomiting
Foreseen user group
Not provided
Foreseen duration between application(s)
Not provided
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Ondansetron
Class(es)
Antiemetics
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Not provided
Foreseen user group
Not provided
Foreseen duration between application(s)
Not provided
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Thalidomide derivates
Class(es)
Immunomodulatory Imides
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Not provided
Foreseen user group
Not provided
Foreseen duration between application(s)
Not provided
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Patent info
Description
Treatment of Vomiting and Nausea with Minimum Dose of Olanzapine
Brief description
Compositions, devices, and methods for transdermal administration of olanzapine and uses thereof, e.g., to treat nausea and vomiting.
Representative patent
US20240189251A1
Category
Formulation
Patent holder
Starton Therapeutics, Inc.
Exclusivity
Not provided
Expiration date
February 20, 2044
Status
Granted
Description
Transdermal delivery of dronabinol
Brief description
Provided is a transdermal drug delivery system comprising dronabinol. The dronabinol transdermal delivery system provides a drug plasma concentration at predetermined rate for a predetermined period of time, offering a simplified therapeutic regimen by decreasing dosing frequency for the treatment and/or prevention of nausea and/or vomiting associated with, for example, chemotherapy.
Representative patent
US20210236417A1
Category
Formulation
Patent holder
Starton Therapeutics, Inc.
Exclusivity
Not provided
Expiration date
April 7, 2041
Status
Granted
Description
Ondansetron In-adhesive Transdermal Patch
Brief description
Drug-in-adhesive transdermal patches for the administration of the ondansetron are described. The patches find use, for example, to treat chemotherapy induced nausea and vomiting (CINV), which requires a high initial flux in the acute phase that is sustained over multiple days or weeks. Embodiments of the disclosure relate in part to formulations for manufacturing self-adhesive patches that include various combinations of a variety of advantageous components, which can be tailored to treat various patient groups or symptoms.
Representative patent
WO2020118091A!
Category
Formulation
Patent holder
Starton Therapeutics, Inc.
Exclusivity
Not provided
Expiration date
December 5, 2019
Status
Granted
Description
Transdermal drug delivery systems for administration of a therapeutically effective amount of lenalidomide and other immunomodulatory agents
Brief description
Transdermal drug delivery systems and methods of fabricating such systems are provided. The active pharmaceutical ingredient can be lenalidomide or other immunomodulatory agents. More particularly, the present invention is directed to improving the solubility of lenalidomide and other immunomodulatory imide compounds and improving the permeation of such compounds through the skin.
Representative patent
EP4351536A1
Category
Not provided
Patent holder
Starton Therapeutics, Inc.
Exclusivity
Not provided
Expiration date
Not provided
Status
Pending
Supporting material
Publications
There are no publication
Additional documents
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Illustrations
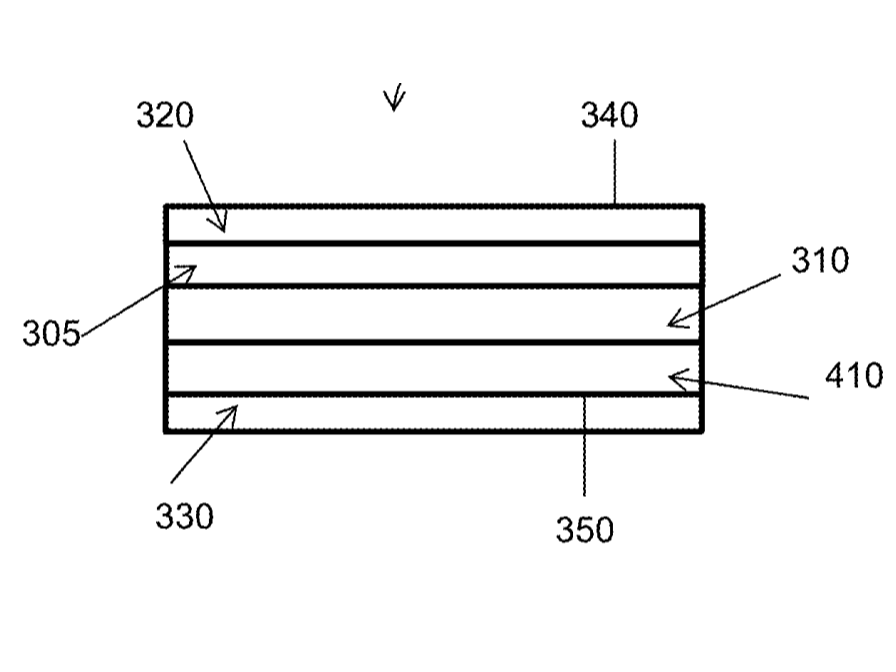
Starsilon Trandermnal patch blueprint
U.S. Patent Application Publication No. 20220395468A1. (2022). Retrieved from https://patentimages.storage.googleapis.com/0c/6d/f6/cf9c3b913cc3e5/US20220395468A1.pdf
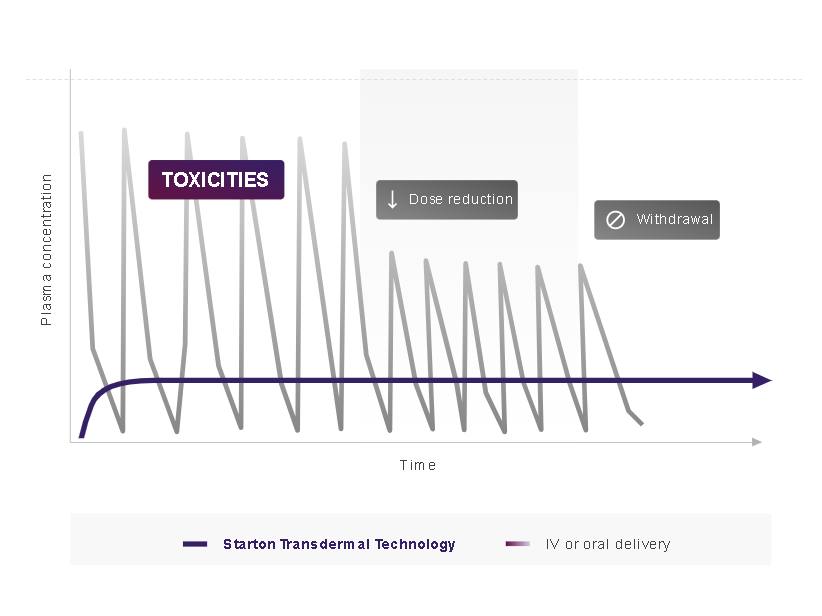
Plasma time curve of Transdermal Drug Delivery System Vs Intravenous
Starton Therapeutics. (n.d.). Pipeline. Retrieved August 8, 2024, from https://www.startontx.com/pipeline/

Starsilon Trandermal Patch
Starton Therapeutics - Precision Blood Cancer Conference September 2021 (March 2015), YouTube. https://www.youtube.com/watch?v=CWzjnNOt934
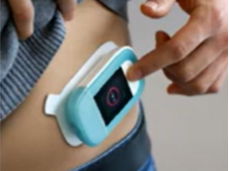
Subcutaneous Pump
Starton Therapeutics - Precision Blood Cancer Conference September 2021 (March 2015), YouTube. https://www.youtube.com/watch?v=CWzjnNOt934