
|
Developed by 

|
Supported by 

|
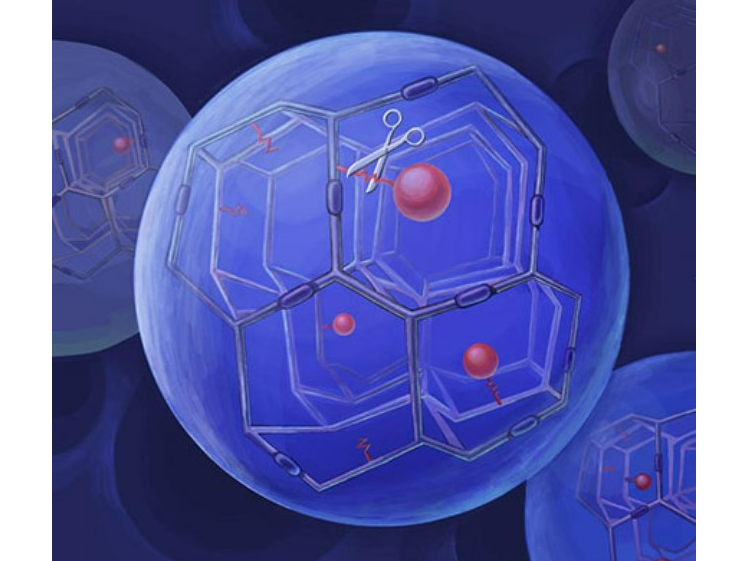
Hydrogel Microspheres with β-eliminative Linker Technology
Based on public informationDeveloper(s)

|
ProLync, Inc. Originator
https://prolynxinc.com
United States ProLynx is a biotechnology company that specializes in developing long-acting drug delivery systems. Their technology involves attaching therapeutic agents to proprietary linkers that degrade at a controlled rate, allowing for the sustained release of drugs over extended periods. This approach is designed to reduce the frequency of dosing and improve patient compliance. |
Sponsor(s)
|
No sponsor indicated |
Partnerships

|
Daiichi Sankyo https://www.daiichisankyo.com/ |

|
Bayer HealthCare LLC https://www.bayer.com/en/ |

|
GlaxoSmithKline https://www.gsk.com/ |
Technology information
Type of technology
Polymer-based particles, PEGylated hydrogel and beta-eliminative cross-linker
Administration route
Subcutaneous, Intravenous, Intratumoral
Development state and regulatory approval
Topoisomerase 1 inhibitors (TOP1i)
Phase II
Not provided
Description
ProLynx's platform leverages custom-synthesized chemical linkers to conjugate therapeutic molecules to carrier systems, thereby enabling controlled and sustained drug release. The biodegradable nature of these linkers, which degrade at predefined rates determined by their molecular architecture, facilitates predictable and prolonged therapeutic efficacy. This technology is versatile, accommodating a broad spectrum of therapeutic modalities, including small molecules, peptides, and proteins.
Technology highlight
1) Drug release in the Prolynx formulation is mediated by β-eliminative linkers, which undergo a rate-controlled elimination reaction. 2) Each linker within the library incorporates a unique “modulator” that independently regulates the drug release kinetics, irrespective of enzymatic influence. 3) The carrier system is composed of circulating macromolecules, such as polyethylene glycol (PEG), fabricated into 40 µm porous PEG-hydrogel microspheres. 4) To synchronize drug release with the degradation of the polymeric matrix, slower-cleaving linkers are integrated into the hydrogel crosslinks.
Technology main components
1) One or more API 2) Cross linker - it has a functional group that reacts with the reactive polymer and a moiety that cleaves by elimination under physiological conditions also comprising a functional group that reacts with one or more polymers. 3) PEGlated hydrogel microspheres 4) Gel-forming solvent (eg: water, alcohols, acetonitrile, or tetrahydrofuran)
Information on the raw materials sourcing, availability and anticipated price
Not provided
Delivery device(s)
No delivery device
APIs compatibility profile
API desired features
Not provided
Additional solubility data
Not provided
Additional stability data
Not provided
API loading: Maximum drug quantity to be loaded
Not provided
API co-administration
Not provided
LogP
Not provided
Scale-up and manufacturing prospects
Scale-up prospects
A large-scale aseptic manufacturing of injectable microsphere is proposed. This unit is capable of preparing up to ∼2 L of high-quality 50 μm diameter hydrogel microspheres/day.
Tentative equipment list for manufacturing
• Reflux Condensers • Magnetic Stirrer • Centrifuge • Filtration Equipment (vacuum filters) • Rotary Evaporator • pH Meter • Oven or Drying Chamber • Cryogenic Freezer
Manufacturing
Manufacturing process requires ISO Class 5 or 7 environment. The manufacturing process includes: • Reflux Apparatus - Crosslinking of Polymers and Incorporation of Drugs • Filtration and Centrifugation Equipment • Spectroscopic Instruments
Specific analytical instrument required for characterization of formulation
• Size Exclusion Chromatography (SEC) • Dynamic Light Scattering (DLS) • Mechanical Testing
Clinical trials
D15-11073
Identifier
NCT02646852
Link
https://clinicaltrials.gov/study/NCT02646852
Phase
Phase I
Status
Completed
Sponsor
ProLynx LLC
More details
This is a Phase I, open-label, two-arm, dose escalation study of PLX038 intravenous infusion administered to patients with refractory or relapsed solid tumors. This study will explore two different dosing schedules: Arm 1, once every 3 week (q3w), and Arm 2, once weekly for 2 consecutive weeks of a 4-week cycle.
Purpose
A Phase I Study of PLX038 in Patients With Advanced Solid Tumors
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2016-03-01
Anticipated Date of Last Follow-up
2023-07-11
Estimated Primary Completion Date
Not provided
Estimated Completion Date
Not provided
Actual Primary Completion Date
2023-02-01
Actual Completion Date
2023-07-01
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Patients must have histologically (or cytologically)-confirmed diagnosis of solid tumor, refractory after standard therapy for the disease or for which conventional systemic therapy is not reliably effective or no effective therapy is available. * Aged ≥ 18 years. * ECOG Performance Status of 0 or 1. * Adequate clinical laboratory values defined as: * absolute neutrophil count ≥ 1.5 × 10\^9/L * platelets ≥ 100 × 10\^9/L * hemoglobin ≥ 9.0 g/dL (transfusions permissible) * plasma creatinine ≤ 1.5 × upper limit of normal (ULN) for the institution or calculated clearance ≥ 60 mL/min (Cockcroft-Gault formula) * bilirubin ≤ 1.5 × ULN * alanine transaminase (ALT) and aspartate transaminase (AST) \< 2.5 × ULN (\< 5 x ULN if documented hepatic metastases)
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
40
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
TOPOLOGY
Identifier
NCT06162351
Link
https://clinicaltrials.gov/study/NCT06162351
Phase
Phase II
Status
Recruiting
Sponsor
Institut Curie
More details
Single arm phase II study for with primary objective to evaluate the efficacy of PLX038 on response rate for patients with pretreated, metastatic or locally advanced triple negative breast cancer.
Purpose
A Study to Evaluate the Efficacy and Toxicities of PLX038, in Patients With Locally Advanced or Metastatic Triple-negative Breast Cancer
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
Not provided
Actual Start Date
2024-04-17
Anticipated Date of Last Follow-up
2024-05-16
Estimated Primary Completion Date
2025-08-19
Estimated Completion Date
2026-03-19
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Willing and able to comply with the protocol and provide written informed consent prior to study-specific screening procedures. * Age ≥ 18 years. * Females and males with cytologically or histologically confirmed breast carcinoma (either the primary or metastatic lesions). * Locally advanced or metastatic disease that is not amenable to curative treatment. * Triple negative breast cancer (both ER and PR \<10%, HER2-negative or HER2-low). * Measurable disease (per RECIST version 1.1). * Prior therapy (administered in the neoadjuvant, adjuvant and/or metastatic setting) with an anthracycline, taxane and sacituzumab-govitecan (unless not medically appropriate or contraindicated for the patient). * Received a minimum of two prior cytotoxic chemotherapy regimens for local
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
44
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Studied LA-formulation(s)
Studied route(s) of administration
Use case
Treatment
Key resources
POP-ART
Identifier
NCT06337630
Link
https://clinicaltrials.gov/study/NCT06337630
Phase
Phase I
Status
Not yet recruiting
Sponsor
Institut Curie
More details
Phase I with a dose finding cohort, followed by expansion cohorts in pre-specified tumor types.
Purpose
A Study on Tuvusertib (Oral ATR Inhibitor) in Combination With PLX038 (Topo1 Inhibitor) in Patients With Advanced Solid Tumors
Interventions
Intervention 1
Countries
Sites / Institutions
Not provided
Trials dates
Anticipated Start Date
2024-06-15
Actual Start Date
Not provided
Anticipated Date of Last Follow-up
2024-03-22
Estimated Primary Completion Date
2025-06-15
Estimated Completion Date
2029-02-15
Actual Primary Completion Date
Not provided
Actual Completion Date
Not provided
Studied populations
Age Cohort
- Adults
- Older Adults
Genders
- All
Accepts pregnant individuals
Unspecified
Accepts lactating individuals
Unspecified
Accepts healthy individuals
No
Comments about the studied populations
Inclusion Criteria: * Willing and able to comply with the protocol and provide written informed consent prior to study-specific screening procedures. * Age ≥ 18 years. * Locally advanced or metastatic solid cancer that is not amenable to curative treatment. * Measurable disease (per RECIST version 1.1). * Received a minimum of one and a maximum of six prior cytotoxic chemotherapy regimens for locally advanced or metastatic cancer. * Resolution of chemotherapy and radiation therapy related toxicities to NCI-CTCAE version 5.0 Grade 1 or lower severity, except for stable sensory neuropathy (≤ Grade 2), alopecia (any grade), presence of clinically managed chronic autoimmune AEs from prior immune therapy. * Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. * Adequate orga
Health status
Not provided
Study type
Interventional (clinical trial)
Enrollment
92
Allocation
Not provided
Intervention model
Single group assignment
Intervention model description
Not provided
Masking
Open label
Masking description
Not provided
Frequency of administration
Not provided
Studied LA-formulation(s)
Not provided
Studied route(s) of administration
Not provided
Use case
Not provided
Key resources
Excipients
Proprietary excipients used
No proprietary excipient used
Novel excipients or existing excipients at a concentration above Inactive Ingredients Database (IID) for the specified route of administration
No novel excipient or existing excipient used
Residual solvents used
No residual solvent used
Additional features
Other features of the technology
- Biodegradable
- Drug-eluting
- Monolithic
- Non-removable
Release properties
1) Drug release is governed by a rate-controlled β-elimination mechanism. The drug release rate is directly proportional to the degree of ionization at physiological pH 2) The API is covalently linked to the hydrogel matrix via a cleavable linker. 3) Hydrolysis of this linker liberates the API, increasing plasma drug concentration and the half-life of the drug. Various acidity of the functional group added to the API controls the rate of release from the hydrogel. 4) The controlled release profile is attributed to the slower degradation rate of the hydrogel matrix.
Injectability
A 25 to 30-gauge needle is used for subcutaneous injections. Insert the needle at an angle of 45° or 90° depending on the length of the needle and/or depth of the subcutaneous layer.
Safety
Phase II studies of PLX038 show that it is safe and effective in previously treated advanced breast cancer. It is a conjugated active metabolite of Irinotecan.
Stability
The structure and integrity of hydrogel remain stable in physiological conditions of pH and temperature.
Storage conditions and cold-chain related features
Not provided
Potential application(s)
Therapeutic area(s)
Use case(s)
Use of technology
Ease of administration
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
Frequency of administration
Monthly, Once every 2 weeks
User acceptance
Not provided
Targeted user groups
Age Cohort- Adults
- All
Pregnant individuals
Unspecified
Lactating individuals
Unspecified
Healthy individuals
No
Comment
Not provided
Potential associated API(s)
Exenatide
Class(es)
Antidiabetic
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Diabetes
Foreseen user group
Not provided
Foreseen duration between application(s)
Once a week
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Poly (ADP-ribose) polymerase (PARP) inhibitors (PARPi)
Class(es)
PEG-PARP inhibitor
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Oncology
Foreseen user group
Not provided
Foreseen duration between application(s)
Not provided
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Topoisomerase 1 inhibitors (TOP1i)
Class(es)
Antineoplastic agent - Topoisomerase 1 inhibitor
Development stage
Phase II
Clinical trial number(s)
NCT06162351
Foreseen/approved indication(s)
Advanced Breast Cancer
Foreseen user group
Women more than 18 years old who are previously treated with chemotherapy
Foreseen duration between application(s)
Every three weeks
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Interleukin 15 (IL-15)
Class(es)
Immunotherapy
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Not provided
Foreseen user group
Not provided
Foreseen duration between application(s)
Not provided but the PK study shows that the t1/2 (half life) is approximately 5 days
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
C-type natriuretic peptides (CNP)
Class(es)
Modified type C natriuretic peptide (CNP) analog
Development stage
Pre-clinical
Clinical trial number(s)
Not provided
Foreseen/approved indication(s)
Achondroplasia
Foreseen user group
Not provided
Foreseen duration between application(s)
Every 2 weeks and once monthly
Applications to Stringent Regulatory Authorities (SRA) / regulatory approvals
Not provided
Patent info
Description
Hydrogels with biodegradable crosslinking
Brief description
Hydrogels that degrade under appropriate conditions of pH and temperature by virtue of crosslinking compounds that cleave through an elimination reaction are described. The hydrogels may be used for delivery of various agents, such as pharmaceuticals.
Representative patent
US11454861B2
Category
Formulation
Patent holder
Prolynx LLC
Exclusivity
Not provided
Expiration date
August 30, 2039
Status
Active
Description
Slow-release conjugates of SN-38
Brief description
Conjugates of SN-38 that provide optimal drug release rates and minimize the formation of the corresponding glucuronate are described. The conjugates release SN-38 from a polyethylene glycol through a β-elimination mechanism.
Representative patent
US10342792B2
Category
Not provided
Patent holder
Prolynx LLC
Exclusivity
Not provided
Expiration date
June 14, 2038
Status
Active
Description
Prodrugs and drug-macromolecule conjugates having controlled drug release rates
Brief description
The present invention provides methods and compositions that permit controlled and prolonged drug release in vivo. The compounds are either prodrugs with tunable rates of release, or conjugates of the drug with macromolecules which exhibit tunable controlled rates of release.
Representative patent
US9387254B2
Category
Not provided
Patent holder
Prolynx LLC
Exclusivity
Not provided
Expiration date
March 21, 2034
Status
Active
Description
Conjugates of somatostatin analogues
Brief description
Conjugates of carriers and hydrogels for controlling the biological half-life of somatostatin and its analogs are disclosed.
Representative patent
US10413594B2
Category
Not provided
Patent holder
Prolynx LLC
Exclusivity
Not provided
Expiration date
June 22, 2024
Status
Active
Supporting material
Publications
<p><span style="color: rgb(51, 51, 51);">Henise, J. et al. </span><a href="https://prolynxinc.com/pdf/Henise_SciReports2024.pdf" rel="noopener noreferrer" target="_blank" style="color: rgb(248, 117, 0); background-color: rgb(255, 255, 255);">A Platform Technology for Ultra-Long Acting Intratumoral Therapy</a><span style="color: rgb(51, 51, 51);">. </span><em style="color: rgb(51, 51, 51);">Sci Reports</em><span style="color: rgb(51, 51, 51);"> 14:14000. doi:org/</span><a href="10.1038/s41598-024-64261-8" rel="noopener noreferrer" target="_blank" style="color: rgb(51, 51, 51);">10.1038/s41598-024-64261-8</a><span style="color: rgb(51, 51, 51);">. (2024)</span></p>
Intratumoral (IT) therapy is a powerful method of controlling tumor growth, but a major unsolved problem is the rapidity that injected drugs exit tumors, limiting on-target exposure and efcacy. We have developed a generic long acting IT delivery system in which a drug is covalently tethered to hydrogel microspheres (MS) by a cleavable linker; upon injection the conjugate forms a depot that slowly releases the drug and “bathes” the tumor for long periods. We established technology to measure tissue pharmacokinetics and studied MSs attached to SN-38, a topoisomerase 1 inhibitor. When MS~SN-38 was injected locally, tissues showed high levels of SN-38 with a long half-life of ~ 1 week. IT MS~SN-38 was ~ tenfold more efcacious as an anti-tumor agent than systemic SN-38. We also propose and provide an example that long-acting IT therapy might enable safe use of two drugs with overlapping toxicities. Here, long-acting IT MS~SN-38 is delivered with concurrent systemic PARP inhibitor. The tumor is exposed to both drugs whereas other tissues are exposed only to the systemic drug; synergistic anti-tumor activity supported the validity of this approach. We propose use of this approach to increase efcacy and reduce toxicities of combinations of immune checkpoint inhibitors such as αCTLA-4 and αPD-1
<p><span style="color: rgb(51, 51, 51);">Carreras, C. et al. </span><a href="https://prolynxinc.com/pdf/Carreras_BC2024.pdf" rel="noopener noreferrer" target="_blank" style="color: rgb(248, 117, 0); background-color: rgb(255, 255, 255);">Long-Acting Poly(ADP-ribose) Polymerase Inhibitor Prodrug for Humans.</a><span style="color: rgb(51, 51, 51);"> </span><em style="color: rgb(51, 51, 51);">Bioconjugate Chem</em><span style="color: rgb(51, 51, 51);"> 35(4), 551-558. (2024) </span><a href="https://pubs.acs.org/doi/10.1021/acs.bioconjchem.4c00112" rel="noopener noreferrer" target="_blank">https://pubs.acs.org/doi/10.1021/acs.bioconjchem.4c00112</a></p>
Poly(ADP-ribose) polymerase inhibitors (PARPi) have been approved for once or twice daily oral use in the treatment of cancers with BRCA defects. However, for some patients, oral administration of PARPi may be impractical or intolerable, and a long-acting injectable formulation is desirable. We recently developed a long-acting PEGylated PARPi prodrug, PEG∼talazoparib (TLZ), which suppressed the growth of PARPi-sensitive tumors in mice for very long periods. However, the release rate of TLZ from the conjugate was too fast to be optimal in humans. We prepared several new PEG∼TLZ prodrugs having longer half-lives of drug release and accurately measured their pharmacokinetics in the rat. Using the rates of release of TLZ from these prodrugs and the known pharmacokinetics of free TLZ in humans, we simulated the pharmacokinetics of the macromolecular prodrugs and released TLZ in humans. From several possibilities, we chose two conjugates that could be administered intravenously every 2 weeks and maintain TLZ within its known therapeutic window. We describe situations where the PEG∼TLZ conjugates would find utility in humans and suggest how the intravenously administered long-acting prodrugs could in fact be more effective than daily oral administration of free TLZ
<p><span style="color: rgb(33, 33, 33);">Fontaine, S. D., Carreras, C. W., Reid, R. R., Ashley, G. W., & Santi, D. V. (2023). A Very Long-acting Exatecan and Its Synergism with DNA Damage Response Inhibitors. </span><em style="color: rgb(33, 33, 33);">Cancer research communications</em><span style="color: rgb(33, 33, 33);">, </span><em style="color: rgb(33, 33, 33);">3</em><span style="color: rgb(33, 33, 33);">(5), 908–916. </span><a href="https://doi.org/10.1158/2767-9764.CRC-22-0517" rel="noopener noreferrer" target="_blank" style="color: rgb(33, 33, 33);">https://doi.org/10.1158/2767-9764.CRC-22-0517</a></p>
Exatecan (Exa) is a very potent inhibitor of topoisomerase I and anticancer agent. It has been intensively studied as a single agent, a large macromolecular conjugate and as the payload component of antigen-dependent antibody–drug conjugates. The current work describes an antigen-independent conjugate of Exa with polyethylene glycol (PEG) that slowly releases free Exa. Exa was conjugated to a 4-arm 40 kDa PEG through a β-eliminative cleavable linker. Pharmacokinetic studies in mice showed that the conjugate has an apparent circulating half-life of 12 hours, which reflects a composite of both the rate of renal elimination (half-life ∼18 hours) and release of Exa (half-life ∼40 hours). Remarkably, a single low dose of 10 μmol/kg PEG-Exa—only approximately 0.2 μmol/mouse—caused complete suppression of tumor growth of BRCA1-deficient MX-1 xenografts lasting over 40 days. A single low dose of 2.5 μmol/kg PEG-Exa administered with low but efficacious doses of the PARP inhibitor talazoparib showed strong synergy and caused significant tumor regression. Furthermore, the same low, single dose of PEG-Exa administered with the ATR inhibitor VX970 at doses of the DNA damage response inhibitor that do not affect tumor growth show high tumor regression, strong synergy, and synthetic lethality.
<p><span style="color: rgb(33, 33, 33);">Schneider, E. L., Carreras, C. W., Reid, R., Ashley, G. W., & Santi, D. V. (2022). A long-acting C-natriuretic peptide for achondroplasia. </span><em style="color: rgb(33, 33, 33);">Proceedings of the National Academy of Sciences of the United States of America</em><span style="color: rgb(33, 33, 33);">, </span><em style="color: rgb(33, 33, 33);">119</em><span style="color: rgb(33, 33, 33);">(30), e2201067119.</span><a href=" https://doi.org/10.1073/pnas.2201067119" rel="noopener noreferrer" target="_blank" style="color: rgb(33, 33, 33);"> https://doi.org/10.1073/pnas.2201067119</a></p>
The C-natriuretic peptide (CNP) analog vosoritide has recently been approved for treatment of achondroplasia in children. However, the regimen requires daily subcutaneous injections in pediatric patients over multiple years. The present work sought to develop a long-acting CNP that would provide efficacy equal to or greater than that of vosoritide but require less frequent injections. We used a technology for half-life extension, whereby a drug is attached to tetra-polyethylene glycol hydrogels (tetra-PEG) by β-eliminative linkers that cleave at predetermined rates. These hydrogels-fabricated as uniform ∼60-μm microspheres-are injected subcutaneously, where they serve as a stationary depot to slowly release the drug into the systemic circulation. We prepared a highly active, stable CNP analog-[Gln6,14]CNP-38-composed of the 38 C-terminal amino acids of human CNP-53 containing Asn to Gln substitutions to preclude degradative deamidation. Two microsphere [Gln6,14]CNP-38 conjugates were prepared, with release rates designed to allow once-weekly and once-monthly administration. After subcutaneous injection of the conjugates in mice, [Gln6,14]CNP-38 was slowly released into the systemic circulation and showed biphasic elimination pharmacokinetics with terminal half-lives of ∼200 and ∼600 h. Both preparations increased growth of mice comparable to or exceeding that produced by daily vosoritide. Simulations of the pharmacokinetics in humans indicated that plasma [Gln6,14]CNP-38 levels should be maintained within a therapeutic window over weekly, biweekly, and likely, monthly dosing intervals. Compared with vosoritide, which requires ∼30 injections per month, microsphere [Gln6,14]CNP-38 conjugates-especially the biweekly and monthly dosing-could provide an alternative that would be well accepted by physicians, patients, and patient caregivers.
<p><span style="color: rgb(33, 33, 33);">Schneider, E. L., Henise, J., Reid, R., Ashley, G. W., & Santi, D. V. (2016). Hydrogel Drug Delivery System Using Self-Cleaving Covalent Linkers for Once-a-Week Administration of Exenatide. </span><em style="color: rgb(33, 33, 33);">Bioconjugate chemistry</em><span style="color: rgb(33, 33, 33);">, </span><em style="color: rgb(33, 33, 33);">27</em><span style="color: rgb(33, 33, 33);">(5), 1210–1215. </span><a href="https://doi.org/10.1021/acs.bioconjchem.5b00690" rel="noopener noreferrer" target="_blank" style="color: rgb(33, 33, 33);">https://doi.org/10.1021/acs.bioconjchem.5b00690</a></p>
We have developed a unique long-acting drug-delivery system for the GLP-1 agonist exenatide. The peptide was covalently attached to Tetra-PEG hydrogel microspheres by a cleavable β-eliminative linker; upon s.c. injection, the exenatide is slowly released at a rate dictated by the linker. A second β-eliminative linker with a slower cleavage rate was incorporated in polymer cross-links to trigger gel degradation after drug release. The uniform 40 μm microspheres were fabricated using a flow-focusing microfluidic device and in situ polymerization within droplets. The exenatide-laden microspheres were injected subcutaneously into the rat, and serum exenatide measured over a one-month period. Pharmacokinetic analysis showed a t1/2,β of released exenatide of about 7 days which represents over a 300-fold half-life extension in the rat and exceeds the half-life of any currently approved long-acting GLP-1 agonist. Hydrogel-exenatide conjugates gave an excellent Level A in vitro-in vivo correlation of release rates of the peptide from the gel, and indicated that exenatide release was 3-fold faster in vivo than in vitro. Pharmacokinetic simulations indicate that the hydrogel-exenatide microspheres should support weekly or biweekly subcutaneous dosing in humans. The rare ability to modify in vivo pharmacokinetics by the chemical nature of the linker indicates that an even longer acting exenatide is feasible.
Additional documents
No documents were uploaded
Useful links
There are no additional links
Access principles
|
|
Collaborate for developmentConsider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology Not provided |
|
|
Share technical information for match-making assessmentProvide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit Not provided |
|
|
Work with MPP to expand access in LMICsIn the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing Not provided |
Comment & Information
Illustrations
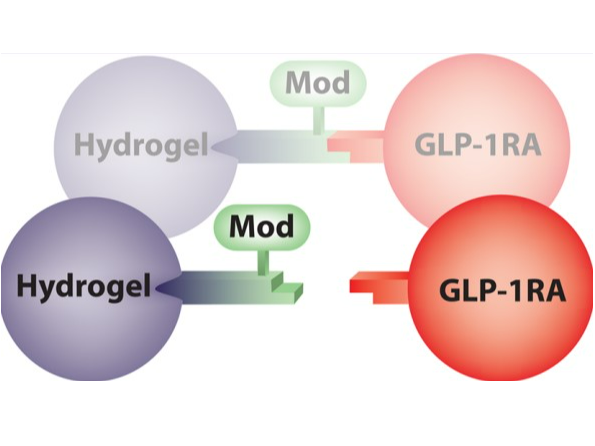
Dissociation of the API from the Hydrogel molecule attached to the cross-linker
ProLynx, Inc. (n.d.). Technology. Retrieved August 16, 2024, from https://www.prolynxinc.com/technology.html
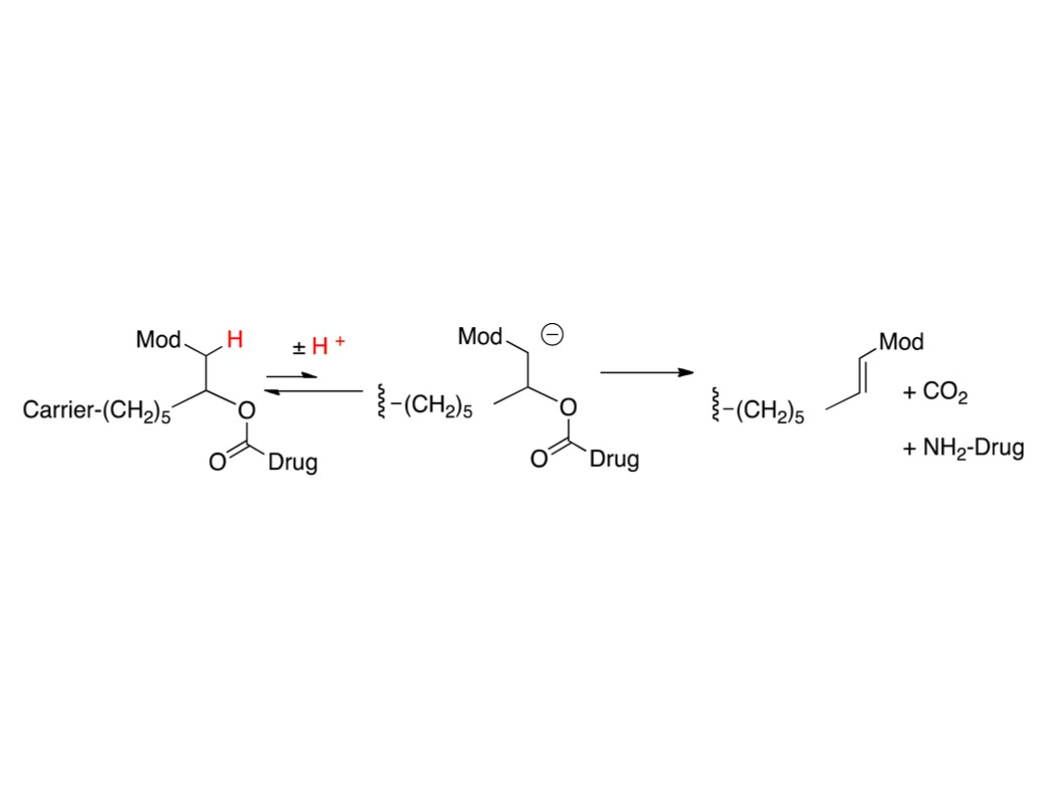
β- elimination of a macromolecular carrier that is attached to a linker that is attached to a drug via a carbamate functional group
Schneider, E. L., Henise, J., Reid, R., Ashley, G. W., & Santi, D. V. (2016). Hydrogel Drug Delivery System Using Self-Cleaving Covalent Linkers for Once-a-Week Administration of Exenatide.
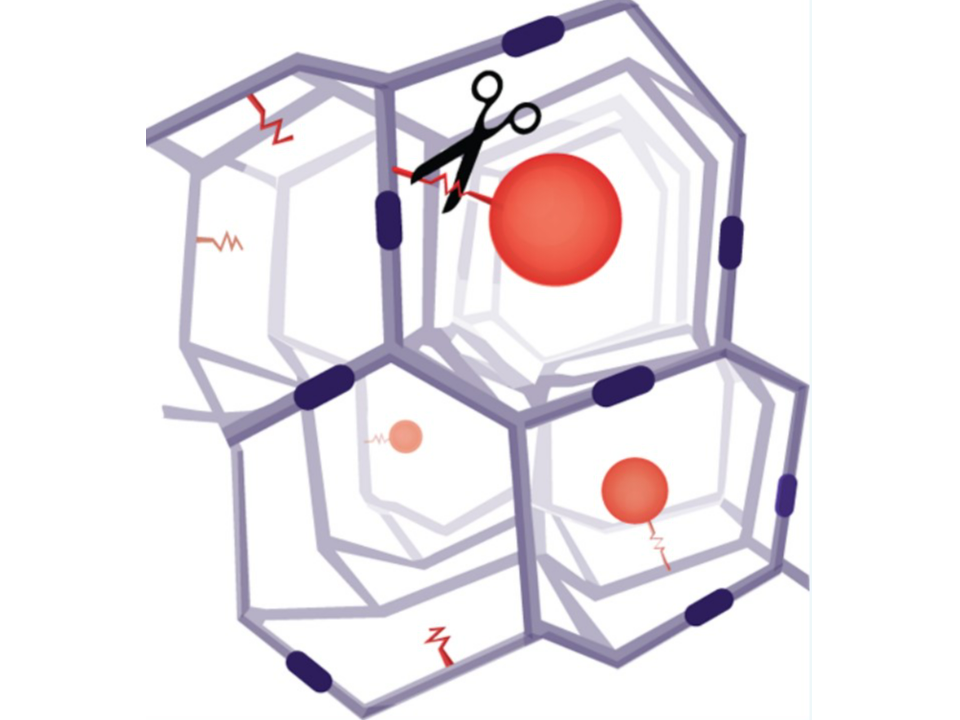
Tetra-PEG hydrogel microspheres by a cleavable β-eliminative linker
Schneider, E. L., Henise, J., Reid, R., Ashley, G. W., & Santi, D. V. (2016). Hydrogel Drug Delivery System Using Self-Cleaving Covalent Linkers for Once-a-Week Administration of Exenatide.

Hydrogel with β- eliminative linker
ProLynx. (n.d.). ProLynx Incorporated. Retrieved August 21, 2024, from https://prolynxinc.com/