Drug information
DSM265
Not provided
Small molecule
DSM265 is an orally-active investigational compound currently in clinical development for the treatment and prevention of malaria. DSM265 functions through selectively inhibiting the dihydroorotate dehydrogenase (DHOPH) enzyme essential for Plasmodium falciparum pyrimidine biosynthesis. Preclinical studies indicated that DSM265 was effective against pre-erythrocytic Plasmodium falciparum and displayed potential as an antimalarial chemoprophylactic due to a favourable pharmacokinetic profile that enables once-weekly dosing. Subsequent in-human clinical trials have shown that while orally administered DSM265 displays high-levels of tolerability and safety, it only induces limited pre-erythrocytic protection, indicating that alternate dosing or combination therapy approaches may be required.
It appears that the development of DSM265 is suspended.
The product did not progress to approval
Therapeutic area(s)
- Malaria
- Pre-Exposure Prophylaxis (PrEP)
- Treatment
Administration route
Oral
Associated long-acting platforms
Oral solid form
Use of drug
- Self-administered
Not provided
Dosage
investigational
Not provided
Not provided
Not provided
Not provided
Not provided
Associated technologies
Not provided
Comment & Information
Developer(s)

MMV is a Swiss-based not-for-profit organization working through a product development partnership model to deliver a portfolio of accessible medicines with the power to treat, prevent and eliminate malaria. We close critical gaps in research, development and access – working “end-to-end” to expand the use of existing antimalarials and innovate new compounds to protect public health.

Takeda aims to discover and deliver life-transforming treatments in our core therapeutic and business areas, including gastrointestinal and inflammation, rare diseases, plasma-derived therapies, oncology, neuroscience and vaccines.
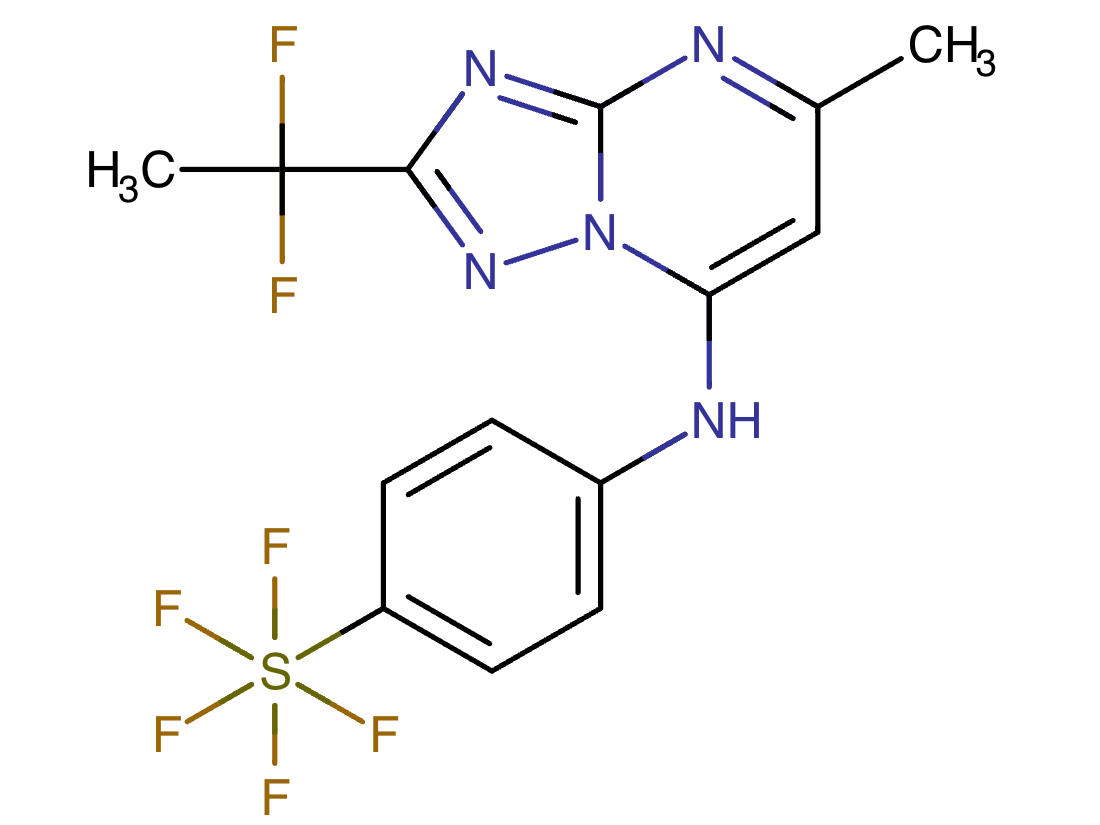
Drug structure
Scale-up and manufacturing prospects
Spray-dried dispersion (SDD) formulations were developed to improve both the solubility and bioavailability of DSM265, in which the drug is present in a stable amorphous state. The spray-drying technology is scalable and suitable for continuous processing, which could potentially facilitate the commercial-scale manufacture of DSM265. Progressive improvements to the chemical synthesis pathway and sourcing of lower-cost starting materials have substantially reduced the overall manufacturing cost of the DSM265 drug substance.
Not provided
The current spray-dried formulation of DSM265 may generate packaging issues due to poor flow properties. Additionally, the oral suspension is administered and dispersed using an aqueous solution containing sweetener and solubilising agents. The requirement for an accompanying dosing medium increases the costs of drug administration and poses commercial as well as logistical challenges. Development of a granulation process to improve the flow properties of the formulation whilst also incorporating the necessary solubilising excipients to enable water-based reconstitution would be beneficial.
Not provided
Excipients
Not provided
Not provided
Not provided
Delivery device(s)
No delivery device
DSM-265 compound
Inhibitors of parasitic dihydroorotate dehydrogenase enzyme (DHOD) are candidate therapeutics for treating malaria. Illustrative of such therapeutic agents include the compound: and a triazolopyrimidine class of compounds that conform to Formula (IX): and their solvates, stereoisomers, tautomers and pharmaceutically acceptable salts. (DSM-265 given as Example 44) Method of treating Malaria.
WO2011041304
Compound
Board of Regents, University of Texas System; Monash University; Medicines For Malaria Venture; University of Washington; Glaxosmithkline Investigacion Y Desarrollo, S.L
Not provided
September 14, 2031
Granted: EP, US, CN, CA, BR, JP, IN, HK
Publications
Alka Marwaha, John White, Farah El_Mazouni, Sharon A Creason, Sreekanth Kokkonda, Frederick S. Buckner, Susan A. Charman, Margaret A. Phillips, and Pradipsinh K. Rathod. Bioisosteric Transformations and Permutations in the Triazolopyrimidine Scaffold To Identify the Minimum Pharmacophore Required for Inhibitory Activity against Plasmodium falciparum Dihydroorotate Dehydrogenase. Journal of Medicinal Chemistry 2012 55 (17), 7425-7436 DOI: www.doi.org/10.1021/jm300351w
Plasmodium falciparum causes approximately 1 million deaths annually. However, increasing resistance imposes a continuous threat to existing drug therapies. We previously reported a number of potent and selective triazolopyrimidine-based inhibitors of P. falciparum dihydroorotate dehydrogenase that inhibit parasite in vitro growth with similar activity. Lead optimization of this series led to the recent identification of a preclinical candidate, showing good activity against P. falciparum in mice. As part of a backup program around this scaffold, we explored heteroatom rearrangement and substitution in the triazolopyrimidine ring and have identified several other ring configurations that are active as PfDHODH inhibitors. The imidazo[1,2-a]pyrimidines were shown to bind somewhat more potently than the triazolopyrimidines depending on the nature of the amino aniline substitution. DSM151, the best candidate in this series, binds with 4-fold better affinity (PfDHODH IC50 = 0.077 μM) than the equivalent triazolopyrimidine and suppresses parasites in vivo in the Plasmodium berghei model.
Jose M. Coteron, María Marco, Jorge Esquivias, Xiaoyi Deng, Karen L. White, John White, Maria Koltun, Farah El Mazouni, Sreekanth Kokkonda, Kasiram Katneni, Ravi Bhamidipati, David M. Shackleford, Iñigo Angulo-Barturen, Santiago B. Ferrer, María Belén Jiménez-Díaz, Francisco-Javier Gamo, Elizabeth J. Goldsmith, William N. Charman, Ian Bathurst, David Floyd, David Matthews, Jeremy N. Burrows, Pradipsinh K. Rathod, Susan A. Charman, and Margaret A. Phillips. Structure-Guided Lead Optimization of Triazolopyrimidine-Ring Substituents Identifies Potent Plasmodium falciparum Dihydroorotate Dehydrogenase Inhibitors with Clinical Candidate Potential. Journal of Medicinal Chemistry 2011 54 (15), 5540-5561. DOI: www.doi.org/10.1021/jm200592f
Drug therapy is the mainstay of antimalarial therapy, yet current drugs are threatened by the development of resistance. In an effort to identify new potential antimalarials, we have undertaken a lead optimization program around our previously identified triazolopyrimidine-based series of Plasmodium falciparum dihydroorotate dehydrogenase (PfDHODH) inhibitors. The X-ray structure of PfDHODH was used to inform the medicinal chemistry program allowing the identification of a potent and selective inhibitor (DSM265) that acts through DHODH inhibition to kill both sensitive and drug resistant strains of the parasite. This compound has similar potency to chloroquine in the humanized SCID mouse P. falciparum model, can be synthesized by a simple route, and rodent pharmacokinetic studies demonstrated it has excellent oral bioavailability, a long half-life and low clearance. These studies have identified the first candidate in the triazolopyrimidine series to meet previously established progression criteria for efficacy and ADME properties, justifying further development of this compound toward clinical candidate status.
Sulyok M et al. “DSM265 for Plasmodium falciparum chemoprophylaxis: a randomised, double blinded, phase 1 trial with controlled human malaria infection.” Lancet Infect Dis. 17(6):636-644 (2017).
Background:
A drug for causal (ie, pre-erythrocytic) prophylaxis of Plasmodium falciparum malaria with prolonged activity would substantially advance malaria control. DSM265 is an experimental antimalarial that selectively inhibits the parasite dihydroorotate dehydrogenase. DSM265 shows in vitro activity against liver and blood stages of P falciparum. We assessed the prophylactic activity of DSM265 against controlled human malaria infection (CHMI).
Methods:
At the Institute of Tropical Medicine, Eberhard Karls University (Tübingen, Germany), healthy, malaria-naive adults were allocated to receive 400 mg DSM265 or placebo either 1 day (cohort 1A) or 7 days (cohort 2) before CHMI by direct venous inoculation (DVI) of 3200 aseptic, purified, cryopreserved P falciparum sporozoites (PfSPZ Challenge; Sanaria Inc, Rockville, MD, USA). An additional group received daily atovaquone-proguanil (250-100 mg) for 9 days, starting 1 day before CHMI (cohort 1B). Allocation to DSM265, atovaquone-proguanil, or placebo was randomised by an interactive web response system. Allocation to cohort 1A and 1B was open-label, within cohorts 1A and 2, allocation to DSM265 and placebo was double-blinded. All treatments were given orally. Volunteers were treated with an antimalarial on day 28, or when parasitaemic, as detected by thick blood smear (TBS) microscopy. The primary efficacy endpoint was time-to-parasitaemia, assessed by TBS. All participants receiving at least one dose of chemoprophylaxis or placebo were considered for safety, those receiving PfSPZ Challenge for efficacy analyses. Log-rank test was used to compare time-to-parasitemia between interventions. The trial was registered with ClinicalTrials.gov, number NCT02450578.
Findings:
22 participants were enrolled between Oct 23, 2015, and Jan 18, 2016. Five participants received 400 mg DSM265 and two participants received placebo 1 day before CHMI (cohort 1A), six participants received daily atovaquone-proguanil 1 day before CHMI (cohort 1B), and six participants received 400 mg DSM265 and two participants received placebo 7 days before CHMI (cohort 2). Five of five participants receiving DSM265 1 day before CHMI and six of six in the atovaquone-proguanil cohort were protected, whereas placebo recipients (two of two) developed malaria on days 11 and 14. When given 7 days before CHMI, three of six volunteers receiving DSM265 became TBS positive on days 11, 13, and 24. The remaining three DSM265-treated, TBS-negative participants of cohort 2 developed transient submicroscopic parasitaemia. Both participants receiving placebo 7 days before CHMI became TBS positive on day 11. The only possible DSM265-related adverse event was a moderate transient elevation in serum bilirubin in one participant.
Interpretation:
A single dose of 400 mg DSM265 was well tolerated and had causal prophylactic activity when given 1 day before CHMI. Future trials are needed to investigate further the use of DSM265 for the prophylaxis of malaria.
Additional documents
No documents were uploaded
Useful links
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing