Type of technology
Aqueous drug particle suspension
Administration route
Subcutaneous, Intravenous
Development state and regulatory approval
Octreotide
Pre-clinical
Not provided
Description
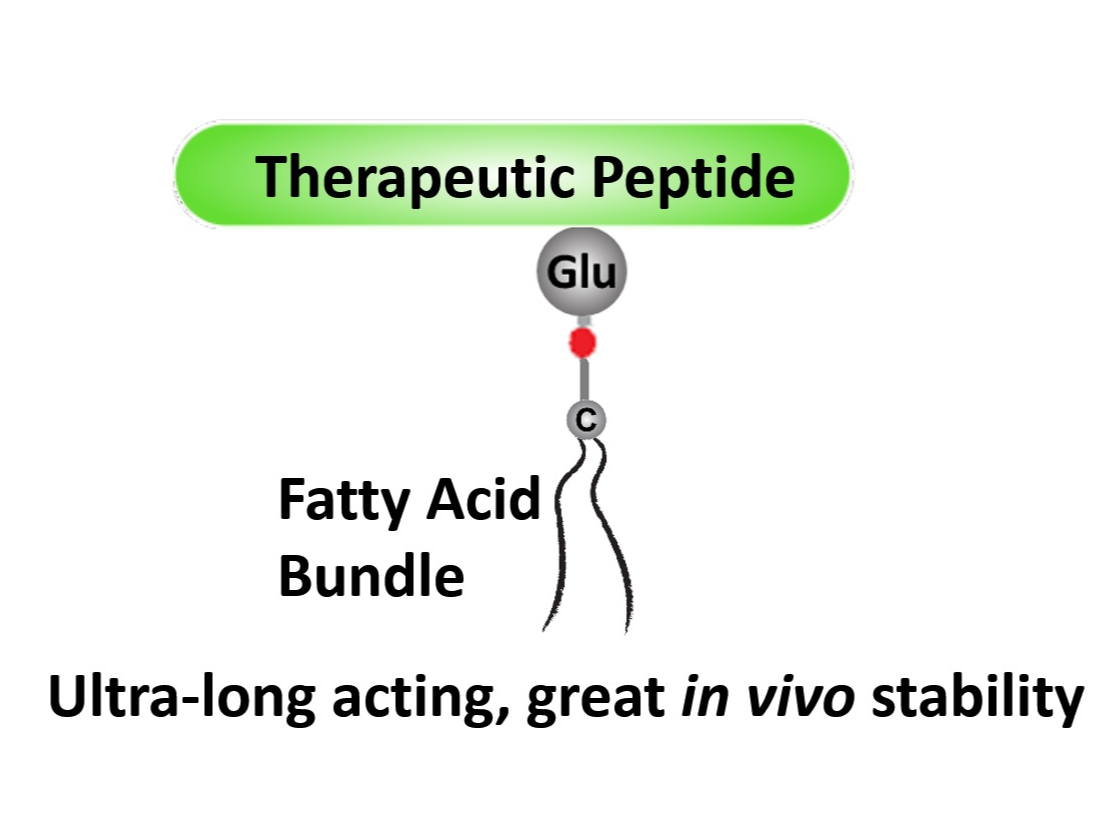
The fatty acid bundles can be employed to modify therapeutic peptides, such as GLP-1, insulin, octreotide, to enhance their association with serum albumin and hence to lengthen their in vivo half-lives. For example, the half-life of octreotide was 1.7 hours in human; after our modification, the half-life is expected to be 2 weeks, which fits a Q4W dose regimen.
Technology highlight
One-step conjugation of fatty acid bundles can significantly improve the in vivo stability and the half-life of therapeutic peptides. The long-acting products with fatty acid bundle modification can be dosed once weekly or monthly, without complicated formulation requirement. The fatty acid bundles are packed multi-arm linker units carrying two or more fatty acid chains, which will enhance the association between drug molecules and serum albumin. Thus, a stronger in vivo stability can be achieved. This technology allows the conjugation of two or more fatty acids onto a specific amino acid residue on the peptide, while maintaining the normal biological interactions with the receptors.
Illustration(s)
Technology main components
The main components is the proprietary "fatty acid bundles", which are composed of our patented multi-arm linkers and fatty acid chains. The fatty acid bundles can be conjugated to therapeutic peptides and significantly improve the in vivo stability and half-life to achieve long-acting properties.
The raw materials are generally available, and the manufacturing process could be transferred to other facilities. For further information, please contact Immunwork, Inc. via bd@immunwork.com
Delivery device(s)
No delivery device
APIs compatibility profile
Min: 100
Unit: mg/mL
Water soluble therapeutic peptides can be modified with our fatty acid bundles.
Therapeutic peptides, such as GLP-1, insulin, octreotide, parathyroid hormone, etc., can be modified with our fatty acid bundles.
Therapeutic peptides, such as GLP-1, insulin, octreotide, parathyroid hormone, etc., can be modified with our fatty acid bundles. Small size proteins (<30 kDa preferred) may also be eligible.
For multiple therapeutic peptides conjugated with our fatty acid bundles, the solubility is between 5 mg/mL to 20 mg/mL in PBS or similar aqueous buffer.
Lyophilized API is very stable under -20 degree Celsius storage.
< 10 wt%
2 different APIs : Our technology modifies API, and the final drug products are in aqueous solution form. Other soluble APIs may be co-administrated.
Not provided
Scale-up and manufacturing prospects
Currently in 100-gram level production for early phase studies. Mass production is feasible.
Not provided
Not provided
Not provided
Excipients
No proprietary excipient used
No novel excipient or existing excipient used
No residual solvent used
Additional features
- Other(s)
Our technology is a modification on API, which is compatible to additional formulation and device technologies.
- Room temperature storage
The preferred administration routes for our current aqueous solution are s.c. and i.v. injection. For s.c. injection, the Cmax can be reached after a few hours to less than two days, depending on the different drug designs.
The API modified with the fatty acid bundle have good solubility, generally 5 - 10 mg/mL for peptide API, in aqueous solutions. It is feasible for various injection methods and devices.
Preliminary preclinical safety assessed. No specific concerns. TE-8105 (long-acting GLP-1 RA): in a study diabetic mice (db/db strain) received bioactive dose (30 nmol/kg, q4d) up to 100 days. In this study, and other studies with shorter treatment duration, no signs of toxicity were observed, in terms of behavior and cage-side observation. TE-8214 (long-acting octreotide): in a study healthy rats received a single dose of 10-time bioactive dose (1 mg/kg), no signs of toxicity were observed, in terms of blood biochemical analyses, CBC, body weight, and cage-side observation.
The stability is still a subject to be further investigated. In our preliminary studies, the drug product stability of TE-8105 (GLP-1) at 4 degree Celsius storage is at least 6 months; stability of TE-8214 (octreotide) at 25 degree Celsius storage is at least 9 months.
The storage conditions are still subjects to be further investigated. Some drug candidates may be suitable for room temperature storage. In our preliminary studies, the drug product stability at 4 degree Celsius storage is at least 6 months. Stability of TE-8214 (octreotide) at 25 degree Celsius storage is at least 9 months.
Therapeutic area(s)
- Other(s) : "Our fatty acid bundle technology can be applied to various therapeutic peptides. In the current stage, metabolic disorders and neuroendocrine tumors are our main focus."
- Diabetes
- Oncology
Not provided
Potential associated API(s)
- Glucagon-like peptide-1 (GLP-1) analogues (GLP-1)
- Insulins
- Octreotide
Use of technology
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
- Self-administered
Weekly, Monthly
Our technology modifies therapeutic peptide APIs. These APIs are soluble in aqueous solution and could be administrated by subcutaneous or intravenous injection. It should be well accepted by users. Pre-fill syringes, implanted pumps, and other dosage form/device are likely applicable to our drug products.
Targeted user groups
- Adults
- All
Unspecified
Unspecified
Unspecified
Not provided
Glucagon-like peptide-1 (GLP-1) analogues (GLP-1)
diabetes treatment, metabolic disorder treatment
Pre-clinical
Not provided
type 2 diabetes, obesity, NASH
Not provided
Two weeks
Not provided
Insulins
diabetes treatment, metabolism disorder treatment
Pre-clinical
Not provided
type 1 diabetes, type 2 diabetes
Not provided
Three days
Not provided
Octreotide
Rare disease treatment, oncology drugs. For example somatostatin analog for acromegaly and neuroendocrine tumor treatment
Pre-clinical
Not provided
Acromegaly, neuroendocrine tumors, carcinoid syndromes
Not provided
Four weeks
Not provided
Publications
There are no publication
Useful links
There are no additional links
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing
Partnerships
No partner indicated
All sponsors
No sponsor indicated
Comment & Information
Immunwork, Inc. has long-acting drug candidates at preclinical stage and looks for licensing and co-developing partners. They are (1) ultra-long-acting octreotide (q4w) for acromegaly and carcinoid syndrome treatment; (2) ultra-long-acting GLP-1 RA (q2w) for type 2 diabetes treatment; (3) ultra-long-acting insulin (q3d) for diabetes treatment. In addition to these drug candidates, we are happy to initiate research collaboration based on our “fatty acid bundle” platform to generate novel ultra-long-acting therapeutic peptides. For more information, please visit: http://www.immunwork.com/