Type of technology
aglycosylated Fc based particles
Administration route
Intravenous, Subcutaneous, Intramuscular
Development state and regulatory approval
efocipegtrutide (GLP-1 triple agonist )
Phase II
Efocipegtrutide Fast Track designation and orphan drug status for the treatment of nonalcoholic steatohepatitis (MASH).
Description
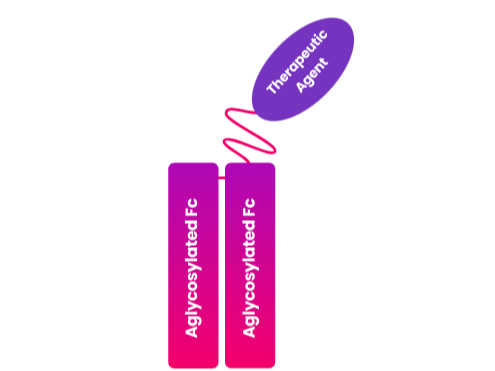
LAPSCOVERY is a long-acting platform technology designed to extend the half-life of biologics administered systemically. The API (therapeutic proteins) is fused with aglycosylated Fc fragments, which enhances the pharmacokinetics of the API. The monomeric Fc fragments help to reduce receptor-mediated clearance, thereby delaying renal filtration and prolonging the therapeutic effect.
Developer(s)

Hanmi Pharmaceutical Ltd., founded in 1973 in South Korea, is a leading R&D-driven company specializing in innovative drug development. It pioneers platform technologies like LAPSCOVERY for long-acting biologics and ORASCOVERY for oral chemotherapeutics. Hanmi collaborates with global firms, including Sanofi, Eli Lilly, and Genentech, advancing oncology, diabetes, and rare disease treatments.
Technology highlight
1. The flexible linker minimizes loss of intrinsic activity 2. Highest bioavailability reduces dose level 3. Low Immunogenicity 4. Minimal Loss of activity 5. Increased cellular solubility 6. No loss of neonatal Fc receptor (FcRn) binding
Illustration(s)
Technology main components
1. Two Aglycolated Fc Fragments 2. Carrier (e.g., Ethylene vinyl acetate) 3. Non-peptide biocompatible polymer (Linker) 4. Excipients—lactose , dextrose , sucrose , sorbitol, mannitol , xylitol , erythritol , maltitol , starch , acacia rubber, alginate , gelatin , calcium phosphate , calcium silicate , cellulose , methyl cellulose , microcrystalline cellulose , polyvinylpyrrolidone, water , methyl hydroxybenzoate , propyl hydroxybenzoate , talc , magnesium stearate , mineral oil , etc. 5. Fillers, anti-coagulant , lubricants, humectants, flavoring agents, preservatives, etc.
Not provided
Delivery device(s)
Single-dose prefilled syringe
APIs compatibility profile
LAPSCOVERY specially focuses on protein-based small molecules designed to target epidermal growth factor receptors (EGFR), including HER2 and HER4, with compounds such as poziotinib,Tuspetinib.
LAPSCOVERY targets a diverse range of proteins, including insulin homologues, human growth hormone, GLP-1 agonists, glucagon, granulocyte colony-stimulating factor, interleukins, and bispecific antibodies.
Not provided
Not provided
50-75 wt%
2 different APIs : Not provided
Not provided
Not provided
Scale-up and manufacturing prospects
Hanmi has a bio plant in Pyeongtaek for the LAPSCOVERY-based biologics occupying a 4,600 m² area.
Not provided
Not provided
Not provided
Excipients
No proprietary excipient used
No novel excipient or existing excipient used
No residual solvent used
Additional features
- Drug-eluting
- At least 1 year shelf life
Upon reaching the target site, the aglycosylated Fc-bound API is released via the detachment of the flexible PEG linker. Once freed, the API binds to its target receptor, eliciting its therapeutic effect through agonistic or antagonistic activity. This monomeric complex partially reduces receptor-mediated clearance and renal filtration. Due to this mechanism, the activity of the API is prolonged.
The prefilled injection of efpeglenatide (LAPScovery formulation) typically uses a 29-gauge needle for subcutaneous administration.
In the phase 3 clinical trial evaluating once-weekly efpeglenatide, the most frequently reported adverse events were mild to moderate gastrointestinal symptoms, including diarrhea, vomiting, and constipation. Treatment-emergent adverse events (TEAEs) occurred in 78.4–83.8% of patients in the treatment group, with two cases of diabetic retinopathy being reported. Serious TEAEs were observed in 9–11% of the patient population.
LASPSCOVERY formulation in its prefilled pen or vial is stable for up to 24 months when stored under the recommended refrigeration condition
Store in a refrigerator at 2°C to 8°C (36°F to 46°F). Do not freeze. It can be stored at room temperature (up to 30°C/86°F) for a maximum of 14 days.
Therapeutic area(s)
- Diabetes
- Other(s) : "Obesity/ metabolic disorders and rare diseases"
- Oncology
Not provided
Potential associated API(s)
- efpegerglucagon (glucagon analog)
- efpeglenatide (exendin-4 analog)
- efocipegtrutide (GLP-1 triple agonist )
- HM16390 (IL-2 analog)
- efpegsomatropin (human growth hormone (hGh) analog)
- sonefpeglutide (Glucagon-like peptide-2 (GLP-2) analogue)
Use of technology
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
Weekly, Monthly
Not provided
Targeted user groups
- Adults
- Older Adults
- All
Unspecified
Unspecified
Unspecified
Not provided
efpegerglucagon (glucagon analog)
Glucagon Analog
Phase II
NCT04732416
Congenital Hyperinsulinism (CHI)
2 years and older
Once weekly
Efpegerglucagon received orphan drug designation from the FDA, EMA, and MFDS for the treatment of CHI
efpeglenatide (exendin-4 analog)
Exendin-4 Analog
Phase III
NCT03353350
Type 2 Diabetes
< 18 years and older
Once weekly
Not provided
efocipegtrutide (GLP-1 triple agonist )
GLP-1 triple agonist
Phase II
NCT04505436
nonalcoholic steatohepatitis (MASH)
Adults ≥ 18 to ≤ 70 years
Once weekly
Efocipegtrutide Fast Track designation and orphan drug status for the treatment of nonalcoholic steatohepatitis (MASH).
HM16390 (IL-2 analog)
Interleukin Analogues
Phase I
NCT06724016
Advanced or Metastatic Solid Tumors
Not provided
Not provided
Not provided
efpegsomatropin (human growth hormone (hGh) analog)
Somatotropin receptor agonists
Phase II
Not provided
Somatotropin deficiency
Not provided
Not provided
Not provided
sonefpeglutide (Glucagon-like peptide-2 (GLP-2) analogue)
GLP-2 analog
Phase II
NCT04775706
Short Bowel Syndrome-associated Intestinal Failure
Not provided
Once monthly
Not provided
Long-acting glucagon conjugate and pharmaceutical composition comprising the same for the prevention and treatment of obesity
Disclosed is a novel long-acting glucagon conjugate in which glucagon or its derivative is covalently linked to a polymer carrier via a non-peptide linker, and a pharmaceutical composition comprising the same as an effective ingredient useful for the prevention and treatment of obesity. Since the long-acting glucagon conjugate of the present invention shows improved in vivo durability and stability, when used in combination with an anti-obesity drug, it is possible to induce synergistic effects on the loss of body weight and decrease in food intake without causing any side-effects such as fluctuation in blood glucose level. Accordingly, the long-acting peptide conjugate of the present invention is very effective for the prevention and treatment of obesity.
US9072794B2
Not provided
Hanmi Science Co Ltd
Not provided
September 28, 2031
Active
Composition for treating diabetes comprising long-acting insulin conjugate and long-acting insulinotropic peptide conjugate
Not provided
Not provided
Not provided
Hanmi Science Co Ltd
Not provided
June 1, 2032
Ceased
Publications
Choi, J., Lee, J., Park, E., Kwon, H., Kim, D., Bae, S., Choi, I. Y., & Kim, H. H. (2023). HM15912, a Novel Long-Acting Glucagon-Like Peptide-2 Analog, Improves Intestinal Growth and Absorption Capacity in a Male Rat Model of Short Bowel Syndrome. The Journal of pharmacology and experimental therapeutics, 384(2), 277–286. https://doi.org/10.1124/jpet.122.001381
Extensive bowel resection caused by various diseases that affect the intestines, such as Crohn's disease, volvulus, and cancer, leads to short bowel syndrome (SBS). Teduglutide is the only approved glucagon-like peptide-2 (GLP-2) drug for SBS; however, it requires daily administration. A novel GLP-2 analog with a prolonged duration of action to reduce dosing frequency and promote a greater efficacy may provide patients with a better quality of life. In the present study, the sustained exposure of HM15912 was characterized in normal male rats. The efficacy of HM15912 on intestinal growth and absorption capacity was also evaluated in normal male mice, rats, and SBS rats. HM15912 exhibited a remarkably extended half-life (42.3 hours) compared with teduglutide (0.6 hours) in rats. Despite somewhat lower in vitro potency on GLP-2 receptor than human GLP-2 or teduglutide, this longer-lasting mode of action promotes HM15912 to be more effective in terms of small intestinal growth than existing GLP-2 analogs even with a less frequent dosing interval of as little as once a week in rodents, including SBS rats. Furthermore, the small intestinal weight was approximately doubled, and the D-xylose absorption was significantly increased after pre-treatment of existing GLP-2 analogs on the market or under clinical development followed by HM15912 in rodents. These results indicate that HM15912 possesses a significant small bowel trophic effect driven by continuously increased exposure, supporting that HM15912 may be a novel treatment option with greater efficacy and the longest dosing interval among existing GLP-2 analogs for SBS with intestinal failure. SIGNIFICANCE STATEMENT: HM15912, a novel long-acting glucagon-like peptide-2 (GLP-2) analog, has a significant small bowel hypertrophic effect in rodents with a reduced frequency of administration compared to the existing GLP-2 analogs on the market or currently under clinical development. This study supports the possibility that HM15912 could be administered much less frequently than other long-acting GLP-2 analogs for patients with short bowel syndrome.
Additional documents
Useful links
There are no additional links
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing