Type of technology
Intra-vaginal ring
Administration route
Topical (Vaginal)
Development state and regulatory approval
MIV-150 (NNRTI) (MIV-150)
Pre-clinical
Not provided
Description
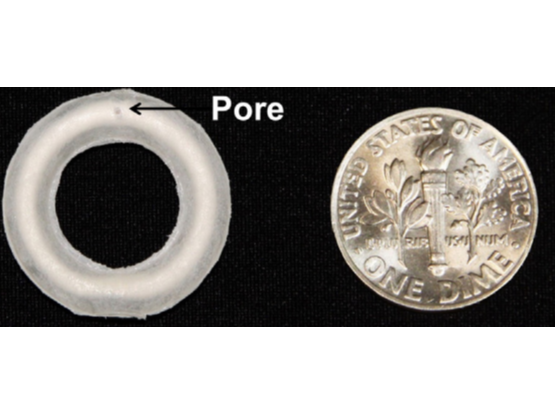
The MZCL intravaginal ring (IVR) is a multipurpose prevention technology delivering a non-nucleoside reverse transcriptase inhibitor (NNRTI), MIV-150, along with zinc acetate dihydrate, carrageenan, and levonorgestrel. This IVR holds therapeutic potential across multiple areas, including the prevention of HIV, HSV-2, and HPV infections, while also providing hormonal contraception. The device measures 20 mm in diameter and 4 mm in thickness, comprising an ethylene-vinyl acetate (EVA) 40 matrix that encapsulates the APIs. To enable controlled release of the APIs, there is a 500μm pore on sides.
Developer(s)

Established in 1952 by John D. Rockefeller III, the Population Council is a nonprofit organization dedicated to biomedical, social science, and public health research. Headquartered in New York City, it operates 18 offices across Africa, Asia, and Latin America, employing nearly 400 staff from 33 countries. The Council has developed contraceptives like the Copper T IUD, Norplant, Jadelle, Mirena.
Technology highlight
1. Multipurpose prevention technology effective against HIV, HSV-2, and HPV. 2. Self-administered for user convenience. 3. Demonstrates superior safety and efficacy in preclinical studies. 4. Functions as a broad-spectrum microbicide and contraceptive.
Illustration(s)
Technology main components
The MZCL intravaginal ring (IVR) contains two regions for controlled release of APIs: the matrix and the core. The matrix, made of translucent ethylene vinyl acetate (EVA-28), holds (i) MIV-150, a non-nucleoside reverse transcriptase inhibitor, and (ii) levonorgestrel, a synthetic progestin for contraception. The core contains (i) carrageenan, a sulfated polysaccharide with antiviral properties, and (ii) zinc acetate, which has antimicrobial activity. This dual-compartment structure enables controlled API release, improving pharmacokinetics and prolonging efficacy. The IVR surface has two pores, 500 μm and 800 μm in diameter, which regulate the release kinetics for sustained drug delivery. This design optimizes therapeutic efficacy for 28 days.
The raw materials for the MZCL IVR are sourced from various manufacturers. Carrageenan is supplied by Industrial Research Limited (Wellington, New Zealand), while zinc acetate is obtained from Spectrum Chemicals (New Brunswick, NJ) and subsequently milled to < 5 μm by Particle Sciences Inc. Levonorgestrel is provided by Crystal Pharma (Valladolid, Spain), and EVA (grade 2803G) is supplied by Celanese (Florence, KY). MIV-150, the anti-HIV agent, is manufactured by Uquifa (Barcelona, Spain). As the MZCL IVR is still under development, it is not yet available for public use.
Delivery device(s)
No delivery device
APIs compatibility profile
Small molecules like non-nucleoside reverse transcriptase inhibitors (NNRTIs) and contraceptive hormones are commonly targeted for inclusion in fixed-dose combination intravaginal rings (IVRs).
Not provided
Not provided
75-90 wt%
4 different APIs : The two small-molecule APIs are embedded in the matrix region of the IVR, whereas the large molecules are immersed in the core region.
Min: 2.9 Max: 6.3
Scale-up and manufacturing prospects
Not provided
Hot melt extruder
The IVR Fabrication includes: (i) Using customized molds, Garner Industries melt-extruded the EVA-28 pellets & precompounded them with MIV-150 (± LNG) at 120 psi and 245°F to produce half intravaginal rings (IVRs) with a central core cavity. (ii) The ZA/CG (3/7) mixture was compressed into the core, and the same matrix was extruded on top to form full MZC or MZCL IVRs. (iii) One half contained the core matrix, the other pure matrix. Target loadings were 3 mg MIV-150, 30 mg ZA, 70 mg CG, and 0.6 mg LNG. Leak tests and pore evaluations (500 μm and 800 μm). (iv) API quantification.
(i) Colorimetic UV assay using Methylene blue (ii) High Performance Liquid Chromatography (HPLC)
Excipients
No proprietary excipient used
Not provided
Not provided
Additional features
- Monolithic
- Removable
- Single-use
- Reservoir-type
- Room temperature storage
- At least 1 year shelf life
The proof-of-concept study demonstrates that the drug release from the vaginal ring occurs at a controlled rate through the pores within the matrix and core regions, delivering the APIs to the vaginal flora. In vitro studies indicate that the MZCL vaginal ring releases APIs over 94 days, while in vivo studies show a release duration of 28 days. Furthermore, the pharmacokinetic profile of the MZCL IVR was found to be dose-independent.
Not applicable
No adverse events (AE) were identified in the preclinical studies of MZCL Intravaginal ring.
ICH Climate Zone IV (A/B)
5 years shelf life This product does not require specific temperature storage conditions. Store in the original package to protect from light.
Therapeutic area(s)
- Disease agnostic
- Contraception
- Other(s) : "HPV and HSV-2"
- HIV
- Pre-Exposure Prophylaxis (PrEP)
- Treatment
Potential associated API(s)
- MIV-150 (NNRTI) (MIV-150)
- Levonorgestrel (LNG)
Use of technology
- Self-administered
Monthly
Not provided
Targeted user groups
- Adults
- Older Adults
- Female
- Cisgender female
- Transgender female
No
No
Yes
Not provided
MIV-150 (NNRTI) (MIV-150)
Non nucleoside Reverse Transcriptase inhibitor
Pre-clinical
Not provided
Prevention of HIV
Not provided
Once monthly
Not provided
Levonorgestrel (LNG)
Hormonal Contraception
Marketed
Not provided
Contraception
Not provided
Once monthly
Not provided
Combination product for the prevention of sexually transmitted infections
Disclosed are compositions for inhibiting transmission of a sexually transmitted infection that contain one or more polyanionic microbicides, such as carrageenans, including lambda carrageenan, as well as water-soluble metal salts and specified lectins. Also disclosed are methods for making and using the compositions.
AU2020203720A1
Not provided
Population Council Inc US Department of Health and Human Services
Not provided
January 13, 2035
Active
Combination therapy intravaginal rings
The present invention provides improved intravaginal drug delivery devices, i.e., intravaginal rings, useful for the prophylactic administration of dapivirine in combination with either an antimicrobial compound or a contraceptive to a human. The present invention also provides methods of blocking DNA polymerization by an HIV reverse transcriptase enzyme, methods of preventing HIV infection in a female human, methods of treating HIV infection in a female human, methods of preventing unintended pregnancy in a female human, and methods of preparing intravaginal rings.
US20220378603A1
Not provided
Population Council Inc
Not provided
November 13, 2034
Active
Carrageenan based antimicrobial compositions
Disclosed are compositions for inhibiting transmission of a sexually transmitted infection that contain one or more carrageenans, including lambda carrageenan. Also disclosed are methods for making and using the compositions.
US20100015247A1
formulation
Population Council Inc
Not provided
Not provided
Abandoned
Publications
Ugaonkar, S. R., Wesenberg, A., Wilk, J., Seidor, S., Mizenina, O., Kizima, L., Rodriguez, A., Zhang, S., Levendosky, K., Kenney, J., Aravantinou, M., Derby, N., Grasperge, B., Gettie, A., Blanchard, J., Kumar, N., Roberts, K., Robbiani, M., Fernández-Romero, J. A., & Zydowsky, T. M. (2015). A novel intravaginal ring to prevent HIV-1, HSV-2, HPV, and unintended pregnancy. Journal of controlled release : official journal of the Controlled Release Society, 213, 57–68. https://doi.org/10.1016/j.jconrel.2015.06.018
Abstract
Women urgently need a self-initiated, multipurpose prevention technology (MPT) that simultaneously reduces their risk of acquiring HIV-1, HSV-2, and HPV (latter two associated with increased risk of HIV-1 acquisition) and prevents unintended pregnancy. Here, we describe a novel core-matrix intravaginal ring (IVR), the MZCL IVR, which effectively delivered the MZC combination microbicide and a contraceptive. The MZCL IVR contains four active pharmaceutical ingredients (APIs): MIV-150 (targets HIV-1), zinc acetate (ZA; targets HIV-1 and HSV-2), carrageenan (CG; targets HPV and HSV-2), and levonorgestrel (LNG; targets unintended pregnancy). The elastomeric IVR body (matrix) was produced by hot melt extrusion of the non-water swellable elastomer, ethylene vinyl acetate (EVA-28), containing the hydrophobic small molecules, MIV-150 and LNG. The solid hydrophilic core, embedded within the IVR by compression, contained the small molecule ZA and the macromolecule CG. Hydrated ZA/CG from the core was released by diffusion via a pore on the IVR while the MIV-150/LNG diffused from the matrix continuously for 94 days (d) in vitro and up to 28 d (study period) in macaques. The APIs released in vitro and in vivo were active against HIV-1ADA-M, HSV-2, and HPV16 PsV in cell-based assays. Serum LNG was at levels associated with local contraceptive effects. The results demonstrate proof-of-concept of a novel core-matrix IVR for sustained and simultaneous delivery of diverse molecules for the prevention of HIV, HSV-2 and HPV acquisition, as well as unintended pregnancy.
Villegas, G., Calenda, G., Ugaonkar, S., Zhang, S., Kizima, L., Mizenina, O., Gettie, A., Blanchard, J., Cooney, M. L., Robbiani, M., Fernández-Romero, J. A., Zydowsky, T. M., & Teleshova, N. (2016). A Novel Microbicide/Contraceptive Intravaginal Ring Protects Macaque Genital Mucosa against SHIV-RT Infection Ex Vivo. PloS one, 11(7), e0159332. https://doi.org/10.1371/journal.pone.0159332
Women need multipurpose prevention products (MPTs) that protect against sexually transmitted infections (STIs) and provide contraception. The Population Council has developed a prototype intravaginal ring (IVR) releasing the non-nucleoside reverse transcriptase inhibitor (NNRTI) MIV-150 (M), zinc acetate (ZA), carrageenan (CG) and levonorgestrel (LNG) (MZCL IVR) to protect against HIV, HSV-2, HPV and unintended pregnancy. Our objective was to evaluate the anti-SHIV-RT activity of MZCL IVR in genital mucosa. First, macaque vaginal tissues were challenged with SHIV-RT in the presence of (i) MIV-150 ± LNG or (ii) vaginal fluids (VF); available from studies completed earlier) collected at various time points post insertion of MZCL and MZC IVRs. Then, (iii) MZCL IVRs (vs. LNG IVRs) were inserted in non-Depo Provera-treated macaques for 24h and VF, genital biopsies, and blood were collected and tissues were challenged with SHIV-RT. Infection was monitored with one step SIV gag qRT-PCR or p27 ELISA. MIV-150 (LCMS/MS, RIA), LNG (RIA) and CG (ELISA) were measured in different compartments. Log-normal generalized mixed linear models were used for analysis. LNG did not affect the anti-SHIV-RT activity of MIV-150 in vitro. MIV-150 in VF from MZC/MZCL IVR-treated macaques inhibited SHIV-RT in vaginal mucosa in a dose-dependent manner (p<0.05). MIV-150 in vaginal tissue from MZCL IVR-treated animals inhibited ex vivo infection relative to baseline (96%; p<0.0001) and post LNG IVR group (90%, p<0.001). No MIV-150 dose-dependent protection was observed, likely because of high MIV-150 concentrations in all vaginal tissue samples. In cervical tissue, MIV-150 inhibited infection vs. baseline (99%; p<0.05). No cervical tissue was available for MIV-150 measurement. Exposure to LNG IVR did not change tissue infection level. These observations support further development of MZCL IVR as a multipurpose prevention technology to improve women's sexual and reproductive health.
Additional documents
No documents were uploaded
Useful links
There are no additional links
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing