Technology name
Tunable Biodegradable Ultra-Long-Acting Polymeric Solid Implant (PSI)
Sponsor(s)
Type of technology
Polymeric implant
Administration route
Subcutaneous
Development state and regulatory approval
Dolutegravir (DTG)
Pre-clinical
None
Description
Ultra-long-acting (ULA) biodegradable polymeric solid implant (PSI) that can accommodate one or more APIs (e.g. ARVs) at translatable human doses in a single implant, in a form of single or multi-layer multi-drug PSI. Administered subcutaneously, PSIs are well tolerated in vivo and effectively delivered drug(s) over 180 days, achieving plasma concentrations above therapeutic targets. While biodegradable, these PSIs can safely be removed to terminate the treatment if required. The versatility of this technology makes it attractive as an ULA drug delivery platform for HIV and other applications.
Developer(s)

Our research at the Benhabbour Lab focuses on engineering novel tunable delivery platforms and polymer-based devices that can treat or prevent a disease. Our work combines the elegance of polymer chemistry with the versatility of engineering and formulation development to design and fabricate efficient and translatable delivery systems for a wide range of applications.
Technology highlight
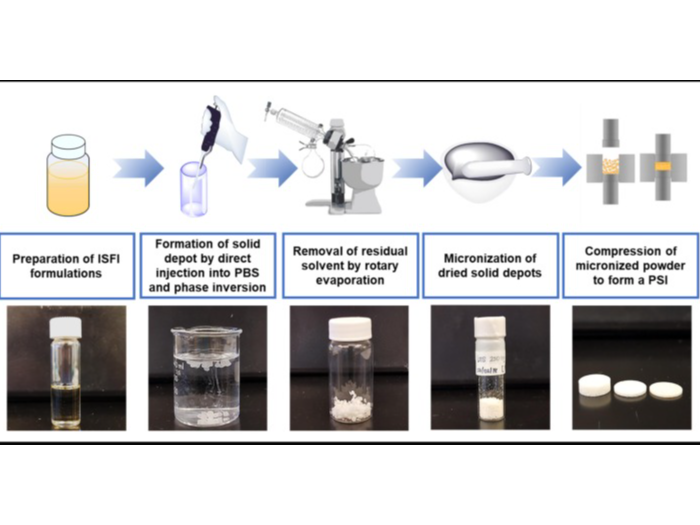
Biodegradable polymeric solid implants (PSIs) are fabricated using phase inversion of drug-loaded polymer-based solution in combination with a compression technique that allows fabrication of PSIs with high drug loading (up to 85 wt%) and compact sizes. The fabrication of these PSIs is accomplished using a simple and scalable stepwise process of (1) phase inversion of a drug-loaded polymer-based solution to form an initial in-situ forming solid implant in an aqueous medium, (2) micronization of dried drug-loaded solid implants, and (3) compression of micronized drug-loaded solid powder. The resulting PSIs are solvent-free and consist of only the biodegradable polymer and drug(s). The manufacturing process does not require high heat or high pressure and can be easily scalable.
Illustration(s)
Technology main components
Poly(DL-lactide-co-glycolide (PLGA) or other biodegradable polymers (e.g. PLA, PCL)
Raw materials are readily available on the market
Delivery device(s)
No delivery device
APIs compatibility profile
Dolutegravir, Rilpivirine, Cabotegravir
Confidential
Confidential
Not provided
Not provided
75-90 wt%
2 different APIs : at least 2
Not provided
Scale-up and manufacturing prospects
Scalability anticipated. Additional information needed.
Additional information needed
New fabrication process using phase inversion and compression. Does not use high heat, high pressure or large volumes of organic solvents. Additional information needed.
Additional information needed
Excipients
No proprietary excipient used
No novel excipient or existing excipient used
Dimethyl Sulfoxide (DMSO)
Additional features
- Biodegradable
- Drug-eluting
- Monolithic
- Removable
- Single-use
- Room temperature storage
- At least 1 year shelf life
Slow diffusion of the drugs through degradation of the PLGA matrix by hydrolysis of ester linkages in the presence of water, with minimal initial burst.
Additional data needed
Well tolerated in vivo (BALB/c mice) over six months. No signs of toxicity, behavioural changes, water consumption, weight loss. Histological staining analysis shows minor inflammation, substantially decreasing 2 weeks after injection. Plasma cytokines showed no systemic acute or chronic inflammation observed.
Additional data needed
Additional data needed
Therapeutic area(s)
- Disease agnostic
- HIV
- HBV
- TB
- COVID 19
- Contraception
- Multipurpose technology : "Prevention of STIs and unplanned pregnancy"
- Pain management
- Oncology
- Diabetes
- Pre-Exposure Prophylaxis (PrEP)
- Treatment
Potential associated API(s)
- Dolutegravir (DTG)
- Rilpivirine (RPV)
- Rilpivirine (RPV) , Dolutegravir (DTG)
- Cabotegravir (CAB)
Use of technology
- Administered by a community health worker
- Administered by a nurse
- To be determined
Once every 6 months
To be determined
Targeted user groups
- Adults
- All
- Male
- Female
- Cisgender female
- Cisgender male
- Transgender female
- Transgender male
- Intersex
- Gender non-binary
Yes
Yes
Unspecified
To be further investigated
Dolutegravir (DTG)
Not provided
Pre-clinical
None
Not provided
Not provided
Not provided
None
Rilpivirine (RPV)
Not provided
Pre-clinical
None
Not provided
Not provided
Not provided
None
Rilpivirine (RPV) , Dolutegravir (DTG)
Antiretrovirals
Pre-clinical
None
HIV PrEP/ART
PLHIV and people at risk of HIV
6 months
None
Cabotegravir (CAB)
Not provided
Not provided
Not provided
Not provided
Not provided
Not provided
Not provided
Publications
Panita Maturavongsadit, Roopali Shrivastava, Craig Sykes, Mackenzie L. Cottrell, Stephanie A. Montgomery, Angela D.M. Kashuba, S. Rahima Benhabbour,
International Journal of Pharmaceutics,
Volume 605, 2021,120844, ISSN 0378-5173,
https://doi.org/10.1016/j.ijpharm.2021.120844.
(https://www.sciencedirect.com/science/article/pii/S0378517321006499)
Lack of adherence is a key barrier to a successful human immunodeficiency virus (HIV) treatment and prevention. We report on an ultra-long-acting (ULA) biodegradable polymeric solid implant (PSI) that can accommodate one or more antiretrovirals (e.g., dolutegravir (DTG) and rilpivirine (RPV)) at translatable human doses (65% wt.) in a single implant. PSIs are fabricated using a three-step process: (a) phase inversion of a drug/polymer solution to form an initial in-situ forming solid implant, (b) micronization of dried drug-loaded solid implants, and (c) compression of the micronized drug-loaded solid powder to generate the PSI. DTG and RPV can be pre-combined in a single PLGA-based solution to make dual-drug PSI; or formulated individually in PLGA-based solutions to generate separate micronized powders and form a bilayer dual-drug PSI. Results showed that in a single or bilayer dual-drug PSI, DTG and RPV exhibited physicochemical properties similar to their pure drug analogues. PSIs were well tolerated in vivo and effectively delivered drug(s) over 180 days with concentrations above 4× PA-IC90 after a single subcutaneous administration. While biodegradable and do not require removal, these PSIs can safely be removed to terminate the treatment if required. The versatility of this technology makes it attractive as an ULA drug delivery platform for HIV and various therapeutic applications.
Keywords: Polymeric solid implants; Long-acting drug delivery; Poly(lactic-co-glycolic acid) (PLGA); Dolutegravir; Rilpivirine; HIV prevention
Maturavongsadit, P., Paravyan, G., Kovarova, M., Garcia, J. V., & Benhabbour, S. R. (2020).
International journal of pharmaceutics: X, 3, 100068.
https://doi.org/10.1016/j.ijpx.2020.100068
We present a long-acting (LA) biodegradable polymeric solid implant (PSI) fabricated using a new process combining in-situ phase inversion and compression. This robust process allows fabrication of solid implants that can have different shapes and sizes, accommodate high drug payloads, and provide sustained drug release over several months. Herein the integrase inhibitor dolutegravir (DTG) was used to develop PSIs for HIV prevention. PSIs were fabricated using a three-step process by (a) phase inversion of DTG-loaded polymer solution to form an initial in-situ forming implant in an aqueous solution, (b) micronization of dried DTG-loaded solid implants, and (c) compression of the micronized DTG-loaded solid implants to form the PSI. High drug loading (up to 85 wt%) was achieved in the PSIs. DTG exhibited minimum burst release in the first 24 h (<6%) and sustained release kinetics over 6 months. The release kinetics of DTG can be fine-tuned by varying drug-loading concentration, the ratio of polymer (poly(lactic-co-glycolic acid), PLGA) to solvent (N-methyl-2-pyrrolidone, NMP) and polymer (PLGA) molecular weight in the precursor solution. The physical/chemical properties of DTG were retained post-storage under accelerated storage conditions (40 °C/75% relative humidity) for 6 months. The versatility of this technology makes it an attractive drug delivery platform for HIV prevention applications.
Keywords: Solid implants, In-situ, Phase inversion, Compression, Long-acting drug delivery, Poly(lactic-co-glycolic acid), HIV prevention
Additional documents
No documents were uploaded
Useful links
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing
Partnerships
Comment & Information
Our research at the Benhabbour Lab focuses on engineering novel tunable delivery platforms and polymer-based devices that can treat or prevent a disease. Our work combines the elegance of polymer chemistry with the versatility of engineering and formulation development to design and fabricate efficient and translatable delivery systems for a wide range of applications including cancer treatment, HIV prevention, osteoporosis and regenerative medicine.
