Drug name
Efruxifermin
Developer(s)
Drug information
Efruxifermin (EFX)
investigational
Biotherapeutic
EFX has been engineered to mimic the biological activity of fibroblast growth factor 21 (FGF21), which regulates multiple metabolic pathways and cellular processes. By delivering sustained and balanced signalling through FGF21’s receptors in liver and adipose tissue, EFX has the potential to treat MASH by addressing all core drivers of disease progression. EFX leverages the whole body to improve metabolic balance. The first Phase 2b study evaluated the efficacy and safety of EFX in patients with pre-cirrhotic MASH, fibrosis stage 2 or 3 (F2-F3). The study met its primary endpoint of ≥1 stage improvement in fibrosis with no worsening of MASH after 24 weeks of treatment for both the 50 mg EFX (41%) and 28 mg EFX (39%) dose groups, compared to 20% for the placebo arm.
Unknown
Unknown
Therapeutic area(s)
- Other(s) : "MASH"
- Treatment
Administration route
Subcutaneous
Associated long-acting platforms
Monoclonal antibodies and antibody drug conjugates
Use of drug
- Administered by a nurse
- Administered by a specialty health worker
- Self-administered
Not provided
Dosage
Not provided
Not provided
Not provided
Not provided
Not provided
Not provided
Associated technologies
Not provided
Comment & Information
Developer(s)
Drug structure
Scale-up and manufacturing prospects
Not provided
Not provided
Not provided
Not provided
Excipients
Not provided
Not provided
Not provided
Delivery device(s)
Not provided
Efruxifermin compositions
Efruxifermin compositions, comprising sugar, arginine/arginine-HCI or arginine/glutamic acid, and a surfactant disclosed in different ratios and concentrations
WO2023064808
Formulation
AKERO THERAPEUTICS, INC.
Not provided
September 11, 2043
Pending: AE, AP, AU, BR, CA, CL, CN, CO, CR, EA, EG, EP, HK, ID, IL, IN, JO, JP, KR, MA, MX, MY, NZ, PA, PE, PH, SA, SG, TH, UA, US, VN, ZA
Method of Treatment using Efruxifermin
Method of treating metabolic disorder (incl. type 1 diabetes, obesity etc.) using Efruxifermin (SEQ ID NO 41)
WO2013033452
Method of treatment
AMGEN INC.
Not provided
August 30, 2032
Granted: AU Pending: JP, MX, US Not in force: EP
Specific Efruxifermin polypeptides
Efruxifermin polypetide sequence specifically claimed as SEQ ID NO 47
WO2010129503
Compound
AMGEN INC.
Not provided
May 4, 2030
Granted: AR, BR, AU, CA, CN, CO, CR, EA (KZ, RU), EP (CH, DE, FR, IT, GB, LI), HK, IN, ID, IL, JP, KR, LB, MY, MX, MA, NZ, PA, GC, PE, PH, SG, ZA, TH, TW, TN, UA, US Pending: BW, EG, JO, LY, VN
Broad Efruxifermin polypeptides
Broad FGF21 mutant polypeptides, covering mutants of Efruxifermin IgG constant domain as SEQ ID NO 13 and Efruxifermin FGF21 as SEQ ID NO 4, and tris(tetraglycylseryl) peptide linker as SEQ ID NO 23 Full Efruxifermin sequence not disclosed
WO2009149171
Compound
AMGEN INC.
Not provided
June 3, 2029
Granted: DZ, AU, BR, CA, CN, CO, EA (KZ, RU), EP (CH, DE, FR, GB, LI), HK, IN, ID, IL, JP, KR, MY, MX, MA, NZ, PE, SG, ZA, TW, TN, UA, US, VN Pending: AR, BW, CL, CR, EG, JO, LY, GC, UY
Publications
Shanaka Stanislaus, Randy Hecht, Junming Yie, Todd Hager, Michael Hall, Chris Spahr, Wei Wang, Jennifer Weiszmann, Yang Li, Liying Deng, Dwight Winters, Stephen Smith, Lei Zhou, Yuesheng Li, Murielle M. Véniant, Jing Xu, A Novel Fc-FGF21 With Improved Resistance to Proteolysis, Increased Affinity Toward β-Klotho, and Enhanced Efficacy in Mice and Cynomolgus Monkeys, Endocrinology, Volume 158, Issue 5, 1 May 2017, Pages 1314–1327, https://doi.org/10.1210/en.2016-1917
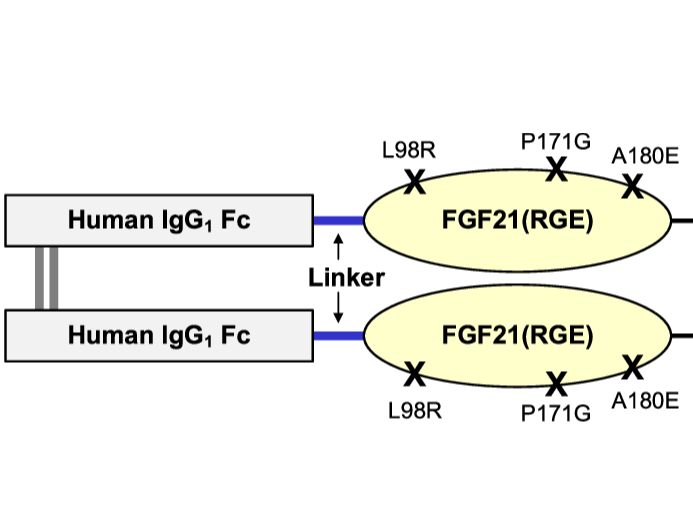
Fibroblast growth factor (FGF) 21 is a natural hormone that modulates glucose, lipid, and energy metabolism. Previously, we engineered an Fc fusion FGF21 variant with two mutations, Fc-FGF21(RG), to extend the half-life and reduce aggregation and in vivo degradation of FGF21. We now describe a new variant developed to reduce the extreme C-terminal degradation and improve the binding affinity to β-Klotho. We demonstrate, by introducing one additional mutation located at the C terminus of FGF21 (A180E), that the new molecule, Fc-FGF21(RGE), has gained many improved attributes. Compared with Fc-FGF21(RG), Fc-FGF21(RGE) has similar in vitro potency, preserves β-Klotho dependency, and maintains FGF receptor selectivity and cross-species reactivity. In vivo, Fc-FGF21(RGE) showed reduced susceptibility to extreme C-terminal degradation and increased plasma levels of the bioactive intact molecule. The circulating half-life of intact Fc-FGF21(RGE) increased twofold compared with that of Fc-FGF21(RG) in mice and cynomolgus monkeys. Additionally, Fc-FGF21(RGE) exhibited threefold to fivefold enhanced binding affinity to coreceptor β-Klotho across mouse, cynomolgus monkey, and human species. In obese and diabetic mouse and cynomolgus monkey models, Fc-FGF21(RGE) demonstrated greater efficacies to Fc-FGF21(RG), resulting in larger and more sustained improvements in multiple metabolic parameters. No increased immunogenicity was observed with Fc-FGF21(RGE). The superior biophysical, pharmacokinetic, and pharmacodynamic properties, as well as the positive metabolic effects across species, suggest that further clinical development of Fc-FGF21(RGE) as a metabolic therapy for diabetic and/or obese patients may be warranted.
Puengel, T., & Tacke, F. (2023). Efruxifermin, an investigational treatment for fibrotic or cirrhotic nonalcoholic steatohepatitis (NASH). Expert Opinion on Investigational Drugs, 32(6), 451–461. https://doi.org/10.1080/13543784.2023.2230115
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most prevalent chronic liver disease and strongly associated with metabolic disorders: obesity, type 2 diabetes (T2D), cardiovascular disease. Persistent metabolic injury results in inflammatory processes leading to nonalcoholic steatohepatitis (NASH), liver fibrosis, and ultimately cirrhosis. To date, no pharmacologic agent is approved for the treatment of NASH. Fibroblast growth factor 21 (FGF21) agonism has been linked to beneficial metabolic effects ameliorating obesity, steatosis, and insulin resistance, supporting its potential as a therapeutic target in NAFLD.
Areas covered
Efruxifermin (EFX, also AKR-001 or AMG876) is an engineered Fc-FGF21 fusion protein with an optimized pharmacokinetic and pharmacodynamic profile, which is currently tested in several phase 2 clinical trials for the treatment of NASH, fibrosis and compensated liver cirrhosis. EFX improved metabolic disturbances including glycemic control, showed favorable safety and tolerability, and demonstrated antifibrotic efficacy according to FDA requirements for phase 3 trials.
Expert opinion
While some other FGF-21 agonists (e.g. pegbelfermin) are currently not further investigated, available evidence supports the development of EFX as a promising anti-NASH drug in fibrotic and cirrhotic populations. However, antifibrotic efficacy, long-term safety and benefits (i.e. cardiovascular risk, decompensation events, disease progression, liver transplantation, mortality) remain to be determined.
Harrison SA, Frias JP, Neff G, et al. Safety and efficacy of once-weekly efruxifermin versus placebo in non-alcoholic steatohepatitis (HARMONY): a multicentre, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol Hepatol. 2023;8(12):1080-1093. doi:10.1016/S2468-1253(23)00272-8
Background: Fibroblast growth factor 21 (FGF21) regulates metabolism and protects cells against stress. Efruxifermin is a bivalent Fc-FGF21 analogue that replicates FGF21 agonism of fibroblast growth factor receptor 1c, 2c, or 3c. The aim of this phase 2b study was to assess its efficacy and safety in patients with non-alcoholic steatohepatitis (NASH) and moderate (F2) or severe (F3) fibrosis.
Methods: HARMONY is a multicentre, randomised, double-blind, placebo-controlled, 96-week, phase 2b trial that was initiated at 41 clinics in the USA. Adults with biopsy-confirmed NASH, defined by a non-alcoholic fatty liver disease activity score (NAS) of 4 or higher and scores of 1 or higher in each of steatosis, ballooning, and lobular inflammation, with histological stage F2 or F3 fibrosis, were randomly assigned (1:1:1), via an interactive response system, to receive placebo or efruxifermin (28 mg or 50 mg), subcutaneously once weekly. Patients, investigators, pathologists, site staff, and the sponsor were masked to group assignments during the study. The primary endpoint was the proportion of patients with improvement in fibrosis of at least 1 stage and no worsening of NASH, based on analyses of baseline and week 24 biopsies (liver biopsy analysis set [LBAS]). A sensitivity analysis evaluated the endpoint in the full analysis set (FAS), for which patients with missing biopsies were considered non-responders. This trial is registered with ClinicalTrials.gov, NCT04767529, and is ongoing.
Findings: Between March 22, 2021, and Feb 7, 2022, 747 patients were assessed for eligibility and 128 patients (mean age 54·7 years [SD 10·4]; 79 [62%] female and 49 male [38%]; 118 [92%] white; and 56 [41%] Hispanic or Latino) were enrolled and randomly assigned to receive placebo (n=43), efruxifermin 28 mg (n=42; two randomised patients were not dosed because of an administrative error), or efruxifermin 50 mg (n=43). In the LBAS (n=113), eight (20%) of 41 patients in the placebo group had an improvement in fibrosis of at least 1 stage and no worsening of NASH by week 24 versus 15 (39%) of 38 patients in the efruxifermin 28 mg group (risk ratio [RR] 2·3 [95% CI 1·1-4·8]; p=0·025) and 14 (41%) of 34 patients in the efruxifermin 50 mg group (2·2 [1·0-5·0]; p=0·036). Based on the FAS (n=128), eight (19%) of 43 patients in the placebo group met this endpoint versus 15 (36%) of 42 in the efruxifermin 28 mg group (RR 2·2 [95% CI 1·0-4·8]; p=0·033) and 14 (33%) of 43 in the efruxifermin 50 mg group (1·9 [0·8-4·3]; p=0·123). The most frequent efruxifermin-related adverse events were diarrhoea (16 [40%] of 40 patients in the efruxifermin 28 mg group and 17 [40%] of 43 patients in efruxifermin 50 mg group vs eight [19%] of 43 patients in the placebo group; all events except one were grade 1-2) and nausea (11 [28%] patients in the efruxifermin 28 mg group and 18 [42%] patients in the efruxifermin 50 mg group vs ten [23%] patients in the placebo group; all grade 1-2). Five patients (two in the 28 mg group and three in the 50 mg group) discontinued due to adverse events. Serious adverse events occurred in four patients in the 50 mg group; one was defined as drug related (ulcerative esophagitis in a participant with a history of gastro-oesophageal reflux disease). No deaths occurred.
Interpretation: Efruxifermin improved liver fibrosis and resolved NASH over 24 weeks in patients with F2 or F3 fibrosis, with acceptable tolerability, supporting further assessment in phase 3 trials.
Additional documents
No documents were uploaded
Useful links
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing