Type of technology
Polymeric implant
Administration route
Intratumoral, ocular insert, intrathecal, Subcutaneous, Intra-vitreal, Topical (Vaginal), Transdermal
Development state and regulatory approval
travoprost
Marketed
Approved by USFDA on 14 December 2023 for open-angle glaucoma (OAG) or ocular hypertension (OHT)
Description
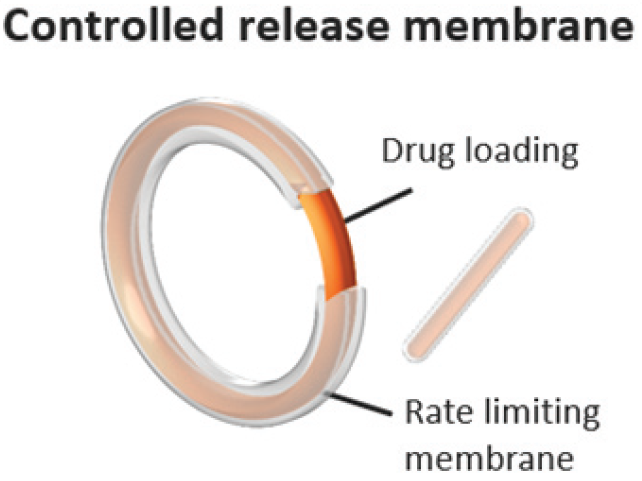
VitalDose is a polymer-based microporous long-acting implant for systemic and local drug delivery. It has a high drug-loading capacity and supports small molecules, peptides, monoclonal antibodies, and iRNA therapeutics. The implant maintains plasma concentrations for months to years, depending on the API’s pharmacokinetics. Available in rod, ring, and film configurations, it enables versatile clinical applications.
Developer(s)

Celanese Corporation, formerly known as Hoechst Celanese, is an American technology and specialty materials company headquartered in Irving, Texas. It is one of the leading global producers of high-performance engineered polymers that are used in a variety of high-value applications.
Technology highlight
1) Contraceptive Sustained Release implant (6 months-5 years) 2) Biodurable design made using hydrophobic polymer 3) Customizable design 4) <70% API loading 5) Zero Order Kinetics 6) Refillable, replaced and retrieved 7) Low off-targeting toxicities 8) 40% vinyl acetate serves as a drug matrix while 18% vinyl acetate forms the rate control membrane
Illustration(s)
Technology main components
1. Polymer Matrix (30–100 wt%) – Core polymers include poly(lactic-co-glycolic acid), polyolefins, polyvinyl chloride, polycarbonates, polysulfones, styrene-acrylonitrile copolymers, polyurethanes, silicone polyether-urethanes, polycarbonate urethanes, and silicone polycarbonate-urethanes. Example: Ateva 4030AC. 2. Excipients—Radiocontrast agents, hydrophilic compounds, bulking agents, plasticizers, surfactants, crosslinking agents, flow aids, colorizing agents (e.g., chlorophyll, methylene blue), antioxidants, stabilizers, lubricants, antimicrobial agents, and preservatives. 3. Surfactants—One or more nonionic, anionic, and/or amphoteric surfactants. 4. API—Small molecules, proteins, or iRNA therapeutics. 5. Bromelain Powder
iDose TR 75 mcg intraocular implant is around 13,950 USD/implant
Delivery device(s)
Sterile single-dose Implant inserter
APIs compatibility profile
VitalDose EVA implants target specific pharmacological classes, including estrogen hormones, tetracycline antibiotics, and antiviral agents. The platform primarily delivers small molecules acting as allosteric agonists or inhibitors. Therapeutic agents that are used for treating solid tumor, ocular inflammation, glaucoma, psychiatric disorders, and osteoporosis are also targeted for VitalDose EVA implant.
Suitable vaccines include whole viral particles, recombinant proteins, subunit proteins (gp41, gp120, gp140), DNA vaccines, plasmids, bacterial vaccines, and polysaccharides such as extracellular capsular polysaccharides, along with other vaccine vectors. Similarly, nucleic acids may include RNA- or DNA-based molecules such as oligonucleotides, aptamers, ribozymes, DNAzymes, and small interfering RNAs (siRNA), including messenger (mRNA), transfer (tRNA), ribosomal (rRNA), and interfering (iRNA) RNAs.
Suitable proteins or peptides may include adrenocorticotropic hormone, angiotensin, β-endorphin, bombesin, calcitonin, calcitonin gene-related polypeptide, cholecystokinin-8, colony-stimulating factors, desmopressin, endothelin, enkephalin, erythropoietins, gastrins, glucagon, human atrial natriuretic polypeptide, interferons, insulin, growth factors, growth hormones, luteinizing hormone-releasing hormone, melanocyte-stimulating hormone, muramyl-dipeptide, neurotensin, oxytocin, parathyroid hormone, peptide T, secretin, and somatomedins.
Not provided
Not provided
50-75 wt%
2 different APIs : Not provided
Min: -6.9 Max: 5
Scale-up and manufacturing prospects
Celanese produces 60,000 metric tons of vinyl acetate annually at its Nanjing facility and plans to expand production by 10,000 metric tons per year. Additionally, Celanese and Nanoform are collaborating to enhance biologics delivery through small implants using the VitalDose platform. Meanwhile, Celanese and Glaat Pharmaceuticals have partnered on formulation development and and clinical trial setup.
HAAKE™ Rheomix OS Mixers
Manufacturing Process of EVA Implants (Hot Melt Extrusion(HME) /Fusion Deposition 3D Modeling) i) Mixing Processes (One or more of the following) 1. Melt mixing 2. Extrusion 3. Batch mixing 4. Roll milling ii) Melt Processing Techniques at 210°C (One or more of the following) 1. Compression molding 2. Injection molding (injection phase and holding phase) 3. Blow molding 4. Vacuum forming 5. Calendaring 6. Extrusion 7. Spinning 9. Film formation The API and ground EVA polymer are dry blended and are fed into HME to yield the final product form.
UV/Vis absorption spectroscopy using Cary 1 split beam instrument
Excipients
No proprietary excipient used
No novel excipient or existing excipient used
No residual solvent used
Additional features
- Biodegradable
- Monolithic
VitalDose is a continuous drug delivery system that releases therapeutic agents from an ethylene vinyl acetate (EVA) membrane at a steady, low-dose rate, with sustained release lasting up to three years. In intratumoral administration, the drug exhibits modified kinetics, allowing for a gradual and controlled release directly into the tumor microenvironment, optimizing therapeutic efficacy while minimizing systemic exposure.
VitalDose is a liquid formulation and can be injected using a standard injection device with a standard 21-25 gauge needle or even thinner depending on the formulation characteristics.
In the Phase 3 clinical trial of iDose, the Travoprost VitalDose implant was evaluated for safety. Among the patients who received the implant, 2.6% experienced systemic serious adverse events (SAEs), while 0.5% experienced ocular SAEs. The most common side effects were iritis, ocular hyperemia, reduced visual acuity, and increased intraocular pressure (IOP).
The API in the EVA implant demonstrated stability over a period of 6 months.
Store at 2°C to 25°C (36°F to 77°F). Do not freeze
Therapeutic area(s)
- Contraception
- Multipurpose technology : "rare diseases, ophthalmology, women’s health, CNS, infectious diseases, and endocrinology applications"
- Oncology
- HIV
- Pre-Exposure Prophylaxis (PrEP)
- Treatment
Potential associated API(s)
- Beta blocking agents
- travoprost
- Dexamethasone
- goserelin
- trastuzumab
Use of technology
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
Monthly, Every 6 months, Yearly
Not provided
Targeted user groups
- Adults
- Older Adults
- Female
- Cisgender female
- Transgender female
- All
No
Unspecified
Unspecified
Not provided
Beta blocking agents
Beta Blocker - Opthalmology
Pre-clinical
Not provided
Glaucoma
Not provided
Not provided
Not provided
travoprost
Prostaglandin analogues
Marketed
NCT03519386
Open-Angle Glaucoma, Ocular Hypertension
Adults < 18years old
Single dose
Approved by USFDA on 14 December 2023 for open-angle glaucoma (OAG) or ocular hypertension (OHT)
Dexamethasone
Synthetic Corticosteroids
Pre-clinical
Not provided
Ocular hypertension
Not provided
Not provided
Not provided
goserelin
Gonadotropin releasing hormone analogues
Pre-clinical
Not provided
Solid Tumors
Not provided
Not provided
Not provided
trastuzumab
HER-2 Inhibitors
Pre-clinical
Not provided
Solid Tumors
Not provided
Not provided
Not provided
Implantable Device for Sustained Release of a Macromolecular Drug Compound
An implantable device for delivery of a macromolecular drug compound is provided. The device comprises a core having an outer surface and a membrane layer positioned adjacent to the outer surface of the core. The core comprises a core polymer matrix within which is dispersed a drug compound having a molecular weight of about 0.5 kDa or more, the polymer matrix containing a hydrophobic polymer. Further, the membrane layer comprises a membrane polymer matrix within which the macromolecular drug compound is optionally dispersed. The concentration of the macromolecular drug compound in the core is greater than the concentration of the macromolecular drug compound in the membrane layer.
US20190358167A1
Device
Celanese EVA Performance Polymers Corporation
Not provided
May 20, 2039
Active
Injection Molded Medical Devices Made From A High Molecular Weight Polyethylene
A high molecular weight polyethylene polymer is formulated so that the polymer is capable of being injection molded. The polyethylene polymer has a Viscosity Number of greater than about 400 ml/g and has a melt flow rate of greater than about 0.9 g/10 min. The polyethylene polymer is of high purity and is particularly well suited for producing medical products.
US20240262942A1
Device
Celanese International Corp
Not provided
Not provided
Pending
Publications
There are no publication
Useful links
There are no additional links
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing
All sponsors
No sponsor indicated