Drug name
Dapivirine (DPV)
Developer(s)
Drug information
Not provided
Dapivirine
Not provided
Small molecule
NNRTI
Dapivirine (DPV) is a diarylpyrimidine non-nucleoside reverse transcriptase inhibitor (NNRTI) marketed as a monthly intravaginal silicone ring for the prevention of HIV-1 infection. NNRTIs function by mechanistically binding to the HIV reverse transcriptase enzyme, thereby inhibiting viral replication. Other topical microbicide formulations of dapivirine include film and gel products that are applied directly to the rectum and/or vagina to prevent the sexual transmission of HIV. Long-acting DPV intravaginal rings are constructed from a malleable silicone elastomer matrix that releases DPV over a 28-day period. DPV intravaginal rings are self-inserted and provide women with a discreet HIV PrEP option that reduces the risk of HIV infection by 31-37% (IPM 027 & ASPIRE).
Dapivirine Vaginal Monthly Ring 25mg has received a positive opinion from European Union and it has received recommendation for its use outside EU. DVR has been approved as a HIV PrEP to reduce the risk of a woman getting infected with HIV-1 through vaginal intercourse in African countries such as South Africa, Zimbabwe, Malawi, Botswana, Zambia, Kenya, Uganda, Namibia, Rwanda, Eswatini and Lesotho. However, DVR has been rejected by United States and it is under clinical hold in United Republic of Tanzania.
Dapivirine Vaginal Ring 25mg monthly is a non-nucleoside reverse transcriptase inhibitor that was first approved by South African Health Products Regulatory Authority (SAHPRA) followed by other African regulatory bodies. DVR is indicated for use by cisgender women as a PrEP to reduce the risk of a woman getting infected by HIV-1 during vaginal intercourse.
Therapeutic area(s)
- HIV
- Pre-Exposure Prophylaxis (PrEP)
Administration route
Topical (Vaginal)
Associated long-acting platforms
Intra-vaginal ring
Use of drug
- Administered by a community health worker
- Administered by a nurse
- Self-administered
- Other/Variable/Unknown : once a month
Not provided
Dosage
25 mg of dapivirine in the monthly ring
25 mg
Not provided
Not provided
Not provided
Associated technologies
3D printed intravaginal ringsVaginal Film (MATRIX)
Dapivirine Monthly Vaginal Ring
Comment & Information
Developer(s)

Janssen Pharmaceuticals
Janssen Pharmaceuticals is a subsidiary company of Johnson & Johnson headquartered in Beerse, Belgium. They focus on manufacturing and developing pharmaceutical products for use in areas such as, Immunology, Infectious Diseases & Vaccines, Pulmonary Hypertension, Cardiovascular & Metabolism, Oncology, and Neuroscience.
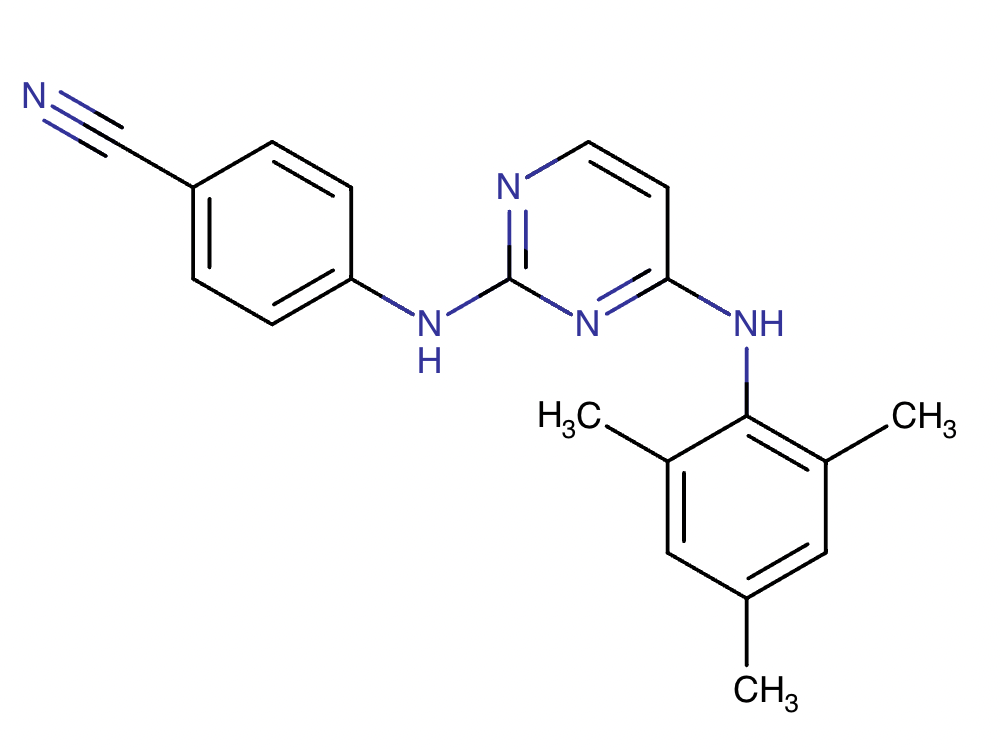
Drug structure
Scale-up and manufacturing prospects
DPV is manufactured commercially through a well-defined synthetic chemical process and undergoes a micronisation procedure to improve drug dispersion within the silicon intravaginal ring. The current DPV ring formulation (Ring-004) contains 25mg of micronised drug product and is produced with NuSil™ DDU-4870 silicone elastomer using addition-curing to prevent the formation of undesirable volatile alcohol by-products. This formulation provides high pharmacokinetic exposure with low overall manufacturing costs. Novel additive manufacturing techniques (e.g. APF) could provide new formulations.
For ring manufacture: Injection molding machine, mixer, automated inspection and packaging machines.
Manufacturing is a non-standard four stage workflow process: (1) preparation of the master batch, (2) pre-mix formation, (3) ring construction using injection addition-curing, and (4) packaging/inspection. For DPV Compound: stability testing indicates that the chemical compound can be stored for up to 6 months at 40ºC and 96 months at 25ºC in the pre-specified commercial packaging. DPV is photo-stable. For Finished Ring Product: stability testing shows that the product can be stored for 48 months at 30ºC. The DPV ring product is photosensitive and should be stored away from light sources.
For DPV Compound: HPLC (determine chemical purity), GC (residual solvents), laser diffraction (determine particle size), Karl Fischer titration (determine water content) and FT-IR & DSC (chemical identification). For Finished Ring Product: HPLC (determine dissolution, content uniformity and degradation products), compression testing machine (determine tensile and compression properties).
Excipients
DDU-4870 is a silicon elastomer product manufactured by NuSil™ Technology Inc. (Carpinteria, CA, USA). In 2016, NuSil Technology, Inc. merged with Avantor.
The novel excipient DDU-4870 is used in the final Ring-004 product formulation. DDU-4870 is contained and packaged within food-grade certified polyethylene pails, which have no effect on excipient quality. At room temperature (25ºC) DDU-4870 is estimated to maintain chemical stability for 12 months.
No residual solvent used
Delivery device(s)
No delivery device
Publications
Welsh, N. R., Malcolm, R. K., Devlin, B., & Boyd, P. (2019). Dapivirine-releasing vaginal rings produced by plastic freeforming additive manufacturing. International Journal of Pharmaceutics. https://doi.org/10.1016/j.ijpharm.2019.118725
Here we report the first use of an additive manufacturing (AM) technique based on high pressure material jetting of molten thermoplastic for the fabrication of dapivirine (DPV) loaded vaginal rings (VRs). The VRs are compared to those produced conventionally using injection molding (IM). VRs (outer diameter 54.0 mm, cross-sectional diameter 4.0 mm) were manufactured by either injection molding or Arburg Plastic Freeforming (APF) - a proprietary droplet deposition modelling (DDM) process, using medical grade thermoplastic polyurethanes (TPUs) loaded with 10% w/w DPV. This unique DDM process was used to produce rings of 100, 50 and 10% matrix infill density. DDM printed VRs with 10% density (57-62 mg drug load) exhibited up to seven-fold increase in DPV release compared to injection molded rings containing 190-194 mg DPV. This work has shown that DDM using the APF technique can be used to manufacture drug delivery devices of varying geometries, densities and surface areas to give precise levels of control over the drug release kinetics. This work presents a new opportunity to increase the release of poorly watersoluble compounds or to achieve desired dosing levels using lower drug loadings than those required using conventional thermoplastic processing techniques.
Malcolm RK, Woolfson AD, Toner CF, Morrow RJ, McCullagh SD. Long-term, controlled release of the HIV microbicide TMC120 from silicone elastomer vaginal rings. J Antimicrob Chemother. 2005 Nov;56(5):954-6. www.doi.org/10.1093/jac/dki326. Epub 2005 Sep 9. PMID: 16155060.
Objectives: The feasibility of providing prolonged and controlled release of the experimental non-nucleoside reverse transcriptase inhibitor TMC120 from a silicone vaginal ring in quantities sufficient to maintain a vaginal concentration offering protection against heterosexual HIV transmission was investigated.
Methods: Core-type, silicone elastomer vaginal rings containing TMC120 were manufactured, and in vitro release studies performed under sink conditions. The experimental release data, as determined by HPLC, were correlated with estimates of vaginal TMC120 concentrations required to inhibit HIV replication.
Results: Continuous, zero-order release of TMC120 from core-type vaginal rings was observed in vitro over a 71 day period, equivalent to 136 μg/day. The release rate is predicted to maintain vaginal concentrations of the antiretroviral in the range of several orders of magnitude in excess of reported HIV inhibitory concentration values.
Conclusions: Continuous and prolonged zero-order release of TMC120 from a silicone vaginal ring device at quantities predicted to prevent HIV infection was observed.
Diarylaniline Derivatives as a Distinct Class of HIV-1 Non-nucleoside Reverse Transcriptase Inhibitors. Bingjie Qin, Xingkai Jiang, Hong Lu, Xingtao Tian, Florent Barbault, Li Huang, Keduo Qian, Chin-Ho Chen, Rong Huang, Shibo Jiang, Kuo-Hsiung Lee, and Lan Xie. Journal of Medicinal Chemistry, 2010, 53 (13), 4906-4916. www.doi.org/10.1021/jm1002952
By using structure-based drug design and isosteric replacement, diarylaniline and 1,5-diarylbenzene-1,2-diamine derivatives were synthesized and evaluated against wild type HIV-1 and drug-resistant viral strains, resulting in the discovery of diarylaniline derivatives as a distinct class of next-generation HIV-1 non-nucleoside reverse transcriptase inhibitor (NNRTI) agents. The most promising compound 37 showed significant EC50 values of 0.003−0.032 μM against HIV-1 wild-type strains and of 0.005−0.604 μM against several drug-resistant strains. Current results also revealed important structure−activity relationship (SAR) conclusions for diarylanilines and strongly support our hypothesis that an NH2 group on the central benzene ring ortho to the aniline moiety is crucial for interaction with K101 of the NNRTI binding site in HIV-1 RT, likely by forming H-bonds with K101. Furthermore, molecular modeling studies with molecular mechanism/general Born surface area (MM/GBSA) technology demonstrated the rationality of our hypothesis.
Additional documents
No documents were uploaded
Useful links
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing