Drug information
Buprenorphine
BUVIDAL; BRIXADI
Small molecule
1. BUVIDAL is a long-acting injectable based on FluidCrystal platform technology used for opioid dependence. 2. Pharmacokinetic parameters: t1/2 - 19 to 25 days; Volume of distribution - 4300 L. 3. The common adverse events in BUVIDAL-treated patients were injection site pain (8.9%), headache (7.5%), constipation (7.5%), nausea (7%), and injection site pruritus (6%).
BRIXADI is approved in the USA whereas BUVIDAL is approved in European countries, Australia, and New Zealand. United Kingdom and Switzerland. Both brands share the same API, excipient and are marketed under Camurus AB.
Approved by US FDA, EMA, TGA, MHRA, Swissmedic and MedSafe.
Therapeutic area(s)
- Substance use disorders
- Treatment
Administration route
Subcutaneous
Associated long-acting platforms
FluidCrystal technology
Use of drug
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
- Weekly
- Monthly
Not provided
Dosage
• BRIXADI (weekly) is available in 8 mg/0.16 mL, 16 mg/0.32 mL, 24 mg/0.48 mL, and 32 mg/0.64 mL; • BRIXADI (monthly) is available in 64 mg/0.18 mL, 96 mg/0.27 mL, and 128 mg/0.36 mL prefilled syringe
40mg/ week; 160 mg/ month
1. The recommended weekly dose in patients not currently receiving buprenorphine treatment is 24 mg of BRIXADI (weekly) titrated up over the first week of treatment (administer a test dose of transmucosal buprenorphine 4 mg when objective signs of mild to moderate withdrawal appear). 2. If needed, during this first week of treatment, administer an additional 8 mg dose of BRIXADI (weekly), waiting at least 24 hours after the previous injection, for a total weekly dose of 32 mg BRIXADI (weekly). 3. Adults who have tolerated a single 4 mg dose of a transmucosal buprenorphine‐containing product. The test dose of transmucosal buprenorphine‐containing product should be administered based on instructions in the appropriate product label.
The recommended starting dose of Buvidal is 16 mg, with one or two additional 8 mg doses at least 1 day apart, to a target dose of 24 mg or 32 mg during the first treatment week. The recommended dose for the second treatment week is the total dose administered during the week of initiation. The maximum dose per week for patients who are on weekly Buvidal treatment is 32 mg with an additional 8 mg dose. The maximum dose per month for patients who are on monthly Buvidal treatment is 160 mg.
Comment & Information
Developer(s)

Camurus AB is a biopharmaceutical company that creates long-acting treatments for serious and chronic diseases. This company originated in 1991. Camurus, based on scientific understanding, aims to enhance patient outcomes through innovative therapies. They specialise in pharmaceutical commercialization and have a portfolio that includes clinical trial products as well as two FDA-approved drugs.
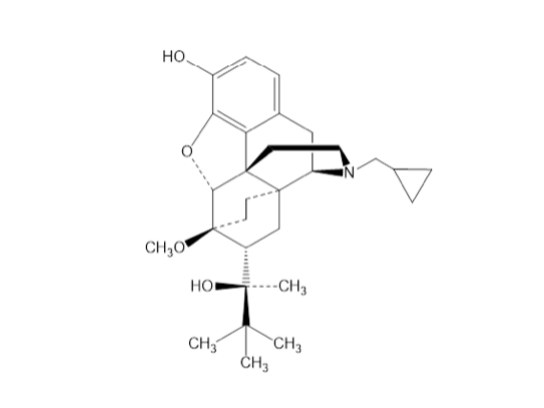
Drug structure
Scale-up and manufacturing prospects
In 2022, 830,000 doses were manufactured
Not provided
• Manufacturing and distribution of Camurus’ products is based on a multi-stage supply chain with several participants involved. Braeburn Pharmaceuticals overlooks the manufacturing of the FDA approved drugs of FluidCrystal in liaison with a third party manufacturer. • The manufacturing process includes: - weighing, dissolving, sterile filtration, filling, closing, visual inspection, labelling, assembly in safety device and secondary packaging. The manufacturing process setup aims to reduce product manufacturing waste by 20% and to increase the use of packaging material originating from susta
The listed analytical instrument used was HPLC with UV detection
Excipients
No proprietary excipient used
• Glycerol dioleate (a lipid) • Ethanol (as solvent) • Phospholipids
No residual solvent used
Delivery device(s)
No delivery device
Depot precursor formulation comprising buprenorphine for sustained delivery of and for treatment of pain or opioid dependence
A depot precursor formulation comprising: a) a controlled-release matrix; b) at least oxygen containing organic solvent; c) at least 12% by weigh of at least one active agent selected from buprenorphine and salts thereof, calculated as buprenorphine free base. Corresponding depot compositions and methods of treatment in pain management, by opioid maintenance and related methods are provided.
WO2014016428
Formulation
Camurus AB
Not provided
July 26, 2033
Granted in: AU, BR, CA, CL, CN, CO, IN, ID, JP, KR, MX, NZ, PE, SG, ZA, TH, US, EA (AM, AZ ,BY, KZ, RU, TJ, TM), EP (AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LI, LT, LU, LV, MC, NL, NO, PL, PT, RO, RS, SE, SI, SK, TR) Pending in: AR, IL, MY, HK
FluidCrystal® injection depot technology, and low viscosity, non-liquid crystalline pre-formulation comprising buprenorphine
he present invention relates to compositions forming a low viscosity mixture of: a. at least one diacyl glycerol and/or at least one tocopherol; b. at least one phospholipid component comprising phospholipids having i. polar head groups comprising more than 50% phosphatidyl ethanolamine, and ii. two acyl chains each independently having 16 to 20 carbons wherein at least one acyl chain has at least one unsaturation in the carbon chain, and there are no more than four unsaturations over two carbon chains; c. at least one biocompatible, oxygen containing, low viscosity organic solvent; wherein optionally at least one bioactive agent is dissolved or dispersed in the low viscosity mixture; and wherein the pre-formulation forms, or is capable of forming, at least one non-lamellar liquid crystall
WO2013083460
Formulation
Camurus AB
Not provided
November 28, 2032
Granted: AU, CA, CN, EA (RU), EP (AT, BE, CH, DE, DK, ES, FI, FR, GB, IE, IS, IT, LI, NL, PL, SE, TR), HK, IL, IN, JP, KR, MX, SG, US, ZA Pending: BR, CO, NZ, PE Not in force: EAPO (AM, AZ, BY, KG, KZ, TJ, TM)
Publications
Vorspan, F., Hjelmström, P., Simon, N., Benyamina, A., Dervaux, A., Brousse, G., Jamain, T., Kosim, M., & Rolland, B. (2019). What place for prolonged-release buprenorphine depot-formulation Buvidal® in the treatment arsenal of opioid dependence? Insights from the French experience on buprenorphine. Expert opinion on drug delivery, 16(9), 907–914. https://doi.org/10.1080/17425247.2019.1649252
Abstract
Introduction: Since the 1990s, opioid maintenance treatments (OMTs), i.e. mostly methadone and buprenorphine, have represented the therapeutic cornerstone of opioid dependence. In France, the public health strategy on opioid dependence, identified here as the 'French model', has consisted of offering a facilitated access to buprenorphine, to reach a large treatment coverage and reduce opioid-related mortality. Areas covered: Recently, a new formulation of subcutaneous buprenorphine depot (Buvidal®) has been approved in Europe for treatment of opioid dependence. The place of Buvidal® among the pre-existing arsenal of OMTs is discussed in the light of the pharmacological specificities of this new formulation, and with the particular standpoint of the French model on opioid dependence. Expert opinion: Buvidal® could constitute a promising treatment option mainly in case of: 1) OMT initiation, including in non-specialized addiction medicine care; 2) Discharge from prison or hospital; Diversion/misuse of 3) buprenorphine or 4) methadone; 5) Clinically stabilized patients wishing to avoid daily oral taking of the medication. As such, this new formulation should be highly accessible, which will require specific pathways through care as the product is intended to be administered by a healthcare professional.
Nunes, E. V., Comer, S. D., Lofwall, M. R., Walsh, S. L., Peterson, S., Tiberg, F., Hjelmstrom, P., & Budilovsky-Kelley, N. R. (2024). Extended-Release Injection vs Sublingual Buprenorphine for Opioid Use Disorder With Fentanyl Use: A Post Hoc Analysis of a Randomized Clinical Trial. JAMA network open, 7(6), e2417377. https://doi.org/10.1001/jamanetworkopen.2024.17377
Importance: Fentanyl has exacerbated the opioid use disorder (OUD) and opioid overdose epidemic. Data on the effectiveness of medications for OUD among patients using fentanyl are limited.
Objective: To assess the effectiveness of sublingual or extended-release injection formulations of buprenorphine for the treatment of OUD among patients with and without fentanyl use.
Design, setting, and participants: Post hoc analysis of a 24-week, randomized, double-blind clinical trial conducted at 35 outpatient sites in the US from December 2015 to November 2016 of sublingual buprenorphine-naloxone vs extended-release subcutaneous injection buprenorphine (CAM2038) for patients with OUD subgrouped by presence vs absence of fentanyl or norfentanyl in urine at baseline. Study visits with urine testing occurred weekly for 12 weeks, then 6 times between weeks 13 and 24. Data were analyzed on an intention-to-treat basis from March 2022 to August 2023.
Intervention: Weekly and monthly subcutaneous buprenorphine vs daily sublingual buprenorphine-naloxone.
Main outcomes and measures: Retention in treatment, percentage of urine samples negative for any opioids (missing values imputed as positive), percentage of urine samples negative for fentanyl or norfentanyl (missing values not imputed), and scores on opiate withdrawal scales and visual analog craving scales.
Results: Of 428 participants, 123 (subcutaneous buprenorphine, n = 64; sublingual buprenorphine-naloxone, n = 59; mean [SD] age, 39.1 [10.8] years; 75 men [61.0%]) had evidence of baseline fentanyl use and 305 (subcutaneous buprenorphine, n = 149; buprenorphine-naloxone, n = 156; mean [SD] age, 38.1 [11.1] years; 188 men [61.6%]) did not have evidence of baseline fentanyl use. Study completion was similar between the fentanyl-positive (60.2% [74 of 123]) and fentanyl-negative (56.7% [173 of 305]) subgroups. The mean percentage of urine samples negative for any opioid were 28.5% among those receiving subcutaneous buprenorphine and 18.8% among those receiving buprenorphine-naloxone in the fentanyl-positive subgroup (difference, 9.6%; 95% CI, -3.0% to 22.3%) and 36.7% among those receiving subcutaneous buprenorphine and 30.6% among those receiving buprenorphine-naloxone in the fentanyl-negative subgroup (difference, 6.1%; 95% CI, -1.9% to 14.1%), with significant main associations of baseline fentanyl status and treatment group. In the fentanyl-positive subgroup, the mean percentage of urine samples negative for fentanyl during the study was 74.6% among those receiving subcutaneous buprenorphine vs 61.9% among those receiving sublingual buprenorphine-naloxone (difference, 12.7%; 95% CI, 9.6%-15.9%). Opioid withdrawal and craving scores decreased rapidly after treatment initiation across all groups.
Conclusions and relevance: In this post hoc analysis of a randomized clinical trial of sublingual vs extended-release injection buprenorphine for OUD, buprenorphine appeared to be effective among patients with baseline fentanyl use. Patients with fentanyl use had fewer opioid-negative urine samples during the trial compared with the fentanyl-negative subgroup. These findings suggest that the subcutaneous buprenorphine formulation may be more effective at reducing fentanyl use.
Trial registration: ClinicalTrials.gov Identifier: NCT02651584.
Additional documents
No documents were uploaded
Useful links
There are no additional links
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing