Type of technology
Based on other organic particles
Administration route
Subcutaneous, Intramuscular, Intra-vitreal, Intravenous, Epidural, Intrathecal, Intraperitoneal
Development state and regulatory approval
Bupivacaine Hydrochloride
Marketed
Exparel has received approval from the United States Food and Drug Administration and marketing authorization from the European Medicines Agency. It is indicated for post-surgical analgesia.
Description
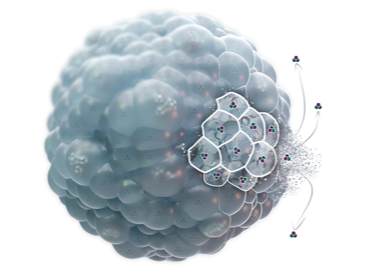
DepoFoam is a lipid-based sustained-release drug delivery system that encapsulates the API into an interconnected network of multi-layered multivesicular liposomes (MVL). These liposomes are highly stable and release the drug from the outermost member of the MVL into the external medium. Exhibiting a microscopic, non-concentric structure, these lipid-based spheroids harbor granular lipid-based particles housing the encapsulated API. The neutral lipid component comprises mono-unsaturated fatty acid ester moieties containing approximately 14–18 carbons, primarily in the form of triglycerides.
Developer(s)

Established in 2007, Pacira Biosciences has surfaced with a mission to furnish non-opioid therapy alternatives for individuals undergoing surgical procedures and experiencing acute pain. Their array of non-opioid injectables derives from their innovative liposomal technology. To amplify their pipeline, Pacira acquired Myoscience Ltd in 2019, fortifying their position in the pain management.
Technology highlight
• The release rate of the API can be modified based on the preparation of the MVL and the neutral lipid component added in the formulation. • The Multivesicular liposomes are biodegradable in nature and biocompatibility for targeted drug delivery at sensitive areas like ocular, epidural, and intrathecal routes of administration. • Optimal concentration at the targeted anatomical location with low systematic exposure.
Illustration(s)
Technology main components
The pMVL formulation contains multi-vesicular liposomes, dextrose, triolein & tricaprylin (mole ratio based on the characteristics of API), phospholipids, DEPC, L-lysine, & DPPG.
The DPPG, triolein, and tricaprylin are obtained from Avanti Polar Lipids, and 10% hydrochloric acid is obtained from Spectrum Chemical. The current cost of the FDA-approved pMVL product Exparel 133 mg (10 mL) dose is 227.63 USD, and Exparel 266 mg (20 mL) dose is 376.12 USD.
Delivery device(s)
No delivery device
APIs compatibility profile
Small molecules in therapeutic areas such as antitumor agents, anaesthetics, analgesics, antimicrobial agents, opiates, hormones etc are considered to be the main choice of interest for pMVL formulation.
Nucleic acids, including DNA, RNA, and antisense oligonucleotides, can be encapsulated using pMVL, and they are selected on a case-by-case basis.
Proteins and peptides, and other compounds like cytokines, hormones (pituitary and hypophyseal hormones), growth factors, vaccines can be encapsulated in the pMVL matrix. Of particular interest are interleukin-2, insulin-like growth factor-1, interferons, insulin, heparin, leuprolide, granulocyte colony Stimulating factor (G-CSF), granulocyte-macrophage colony stimulating factor (GM-CSF), tumor necrosis factor, inhibin, tumor growth factor alpha and beta, Mullerian inhibitory substances, calcitonin, and hepatitis B vaccine.
Not provided
Not provided
≥ 80%
Not provided
Scale-up and manufacturing prospects
The company has expanded to a 200-liter manufacturing suite, which has the capacity to produce up to 200 million doses per year.
Nozzle valve type spray dryer, solvent removal vessel, a solvent removal gas exit orifice comprising a gas outlet tube, three-fluid atomizing nozzle apparatus, continuous-flow emulsification system, continuous processing system, evaporation apparatus, high shear mixer.
• GMP ISO 7 or higher • First Emulsion - API lipid emulsion is formulated volatile water - immiscible solvent and filtered in aseptic environment • H3PO4 solution is made, filtered and mixed with first emulsion to produce w/o emulsion • Second Emulsion - Dextrose Lysin Solution is made, filtered and w/o emulsion is added to this solution to produce w/o/w emulsion is produced • Solvent Extraction - Sparge the prepared w/o/w emulsion in the Sparge Vessel and extract the concentrate • Microfiltration - Microfiltration using Diafiltration vessel via NaCl in a cross-flow filtration system.
• Freeze-fracture electron microscopy • 31P NMR spectroscopy, confocal microscopy to identify the locations of triglycerides in the MVL matrix. • HPLC to identify impurities in the lipid matrix.
Excipients
Not provided
Not provided
Not provided
Additional features
- Biodegradable
- Drug-eluting
- Reservoir-type
- At least 1 year shelf life
The release pattern of API from the pMVL matrix is triphasic. The pMVL technology holds promise in extending the release of the drug to the target site within an effective dosage range, spanning from 26 hours to 90 days, via the liposomal matrix with an initial burst from the surface vesicle within 20 hours of administration.
pMVL preparations can be injected using a 25 gauge or larger-bore needle to ensure the preservation of structural integrity for liposomal bupivacaine particles. Moreover, pMVL products presents diverse injectable formats, including but not limited to Intravenous, Epidural, Intraperitoneal, Subcutaneous, Intramuscular, Ocular, and Intrathecal administrations.
The results of Phase III clinical trials of FDA-approved Exparel (300 mg and 600 mg) showed that there were no severe adverse drug reactions. However, 50-60% of participants experienced common adverse reactions similar to those seen with the generic drug.
Proprietary multivesicular liposome (pMVL) products offer better stability, with the potential to remain viable for up to two years if left unopened.
pMVL formulations are typically refrigerated at temperatures ranging from 2°C to 8°C. They can be stored at a controlled room temperature of 20°C to 25°C for a maximum of 30 days in sealed, intact (unopened) vials. Avoid freezing or exposing the vials to temperatures exceeding 40°C for prolonged periods. Before administration, allow the vials to reach room temperature (20°C to 25°C) for up to 4 hours.
Therapeutic area(s)
- Pain management
- Other(s) : "Lymphomatous meningitis"
- Treatment
Potential associated API(s)
- Bupivacaine Hydrochloride
- Cytarabine
Use of technology
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
Single Dose administration lasting for 72 hours, Weekly
Not provided
Targeted user groups
- Adults
- Older Adults
- All
Yes
Unspecified
Unspecified
Not provided
Bupivacaine Hydrochloride
Anaesthetics
Marketed
NCT05157841
Postsurgical pain management
Patients who are older than 6 years
Single dose administration (lasts up to 72 hours)
Exparel has received approval from the United States Food and Drug Administration and marketing authorization from the European Medicines Agency. It is indicated for post-surgical analgesia.
Cytarabine
Antineoplastic Agent
Marketed
NCT00029523
Lymphomatous meningitis
Patients who are older than 3 years
Every 14 days for 2/3 doses
DepoCyt has been approval in the United States Food and Drug Administration and received marketing authorization from the European Medicines Agency.
Method for Formulating Multivesicular Liposomes
The present invention generally relates to the field of pharmaceutical sciences. More specifically, the present invention relates to an evaporation apparatus and a process for the preparation of multivesicular liposomes (MVL) using such an apparatus and the process of formulating the Liposomal injection.
WO2011127456
Formulation
Pacira Pharmaceuticals
Not provided
April 8, 2031
Not provided
Sustained Release Anesthetic Compositions
The invention provides a method for obtaining local anesthetics encapsulated in liposomes, such as multivesicular liposomes, with high encapsulation efficiency and slow release in vivo. When the encapsulated anesthetic is administered as a single intravenous dose, the duration of anesthesia and the half-life of the drug at the local site of action are increased compared to the injection of unencapsulated anesthetic. The maximum tolerated dose of encapsulated anesthetic is also markedly increased in the liposomal formulation compared to the injection of unencapsulated anesthetic. These results show that the liposomal formulation of local anesthetic is useful for sustained local infiltration and nerve block anesthesia.
USOO8182835B2
Not provided
Pacira Pharmaceuticals, Inc
Not provided
April 1, 2025
Not provided
Publications
Angst, M. S., & Drover, D. R. (2006). Pharmacology of drugs formulated with DepoFoam: a sustained release drug delivery system for parenteral administration using multivesicular liposome technology. Clinical pharmacokinetics, 45(12), 1153–1176. https://doi.org/10.2165/00003088-200645120-00002
Multivesicular liposomes are structurally distinct from lamellar liposomes and consist of an aggregation of hundreds of water-filled polyhedral compartments separated by bi-layered lipid septa. The unique architecture of multivesicular liposomes allows encapsulating drug with greater efficiency, provides robust structural stability and ensures reliable, steady and prolonged drug release. The favourable characteristics of multivesicular liposomes have resulted in many drug formulations exploiting this technology, which is proprietary and referred to as DepoFoam™.
Additional documents
No documents were uploaded
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing
All sponsors
No sponsor indicated