Technology name
Drug Combination Nanoparticles (DcNP)
Sponsor(s)
Type of technology
Based on other organic particles, Aqueous drug particle suspension
Administration route
Subcutaneous
Development state and regulatory approval
Lamivudine (3TC), Dolutegravir (DTG), Tenofovir (TFV)
Phase I
Not provided
Description
A targeted and long-acting Drug combination Nano-Particle (DcNP) platform that enables transformations of existing and new drug entities from short-acting daily doses to a long-acting drug combination for a maximum therapeutic effect through sustained viral suppression.
Developer(s)

Targeted & Long-acting drug Combination (TLC) Program, University of Washington
The purpose of the Targeted Long-acting Combination AntiRetroviral Therapy (TLC-ART) program is to develop one or more safe, stable, scalable and tolerable long acting antiretroviral combinations for treatment of HIV infection.
Technology highlight
TLC's enabling DcNP technology has been validated to work with a number of current HIV drug combinations using clinics in both pediatric and adult populations. A number of these formulations have been evaluated in non-human primate models to provide both plasma and cell drug levels that persist for weeks after a single subcutaneous injection. This technology has been shown to bring together water soluble (such as tenofovir and lamivudine) and insoluble (such as lopinavir, ritonavir, atezenovir, and dolutegravir) drugs into an all-in-one long-acting injectable product. At least 4 formulations have been tested in NHP to have long-acting plasmakinetics for all drugs and are targeted to enhanced drug levels above plasma drug combinations in HIV host cells-PBMCs.
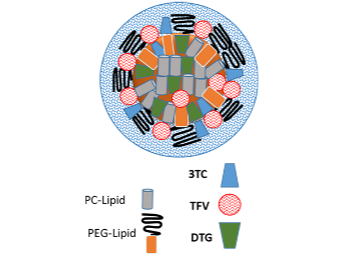
Illustration(s)
Technology main components
Drug API and common lipid excipients used in pharmaceutical formulations.
The existing HIV drug APIs can be sourced through WHO pre-qualified raw material suppliers.
Delivery device(s)
No delivery device
APIs compatibility profile
Tenofovir, Lamivudine, Dolutegravir, Lopinavir, Ritonavir, Atazanavir, Efavirenz
Not provided
Not provided
10-30 wt%
4 different APIs : 2 to 4 different drugs in a single injection are being investigated
Not provided
Scale-up and manufacturing prospects
Good
Spray-dryer; homogenization; size-reduction
Injectable cGMP facilties
Not provided
Excipients
No proprietary excipient used
No novel excipient or existing excipient used
No residual solvent used
Additional features
- Biodegradable
- Non-removable
- Room temperature storage
- At least 1 year shelf life
Intracellular uptake and accessible by cellular enzymes to transform into active metabolites such as TFV-DP and 3TC-TP.
Not provided
Not provided
Not provided
No cold-chain needed
Therapeutic area(s)
- HIV
- Treatment
Potential associated API(s)
- Lamivudine (3TC) , Dolutegravir (DTG) , Tenofovir (TFV)
- Lopinavir and ritonavir (LPV/r) , Tenofovir (TFV)
- Tenofovir-Lamivudine-Dolutegravir (TLD) - long-acting injectable (LAI) (TLD LAI)
Use of technology
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
- Self-administered
Monthly
Not provided
Targeted user groups
- Adults
- Male
- Female
- Cisgender female
- Cisgender male
- Transgender female
- Transgender male
- Intersex
- Gender non-binary
- All
Unspecified
Unspecified
No
Not provided
Lamivudine (3TC) , Dolutegravir (DTG) , Tenofovir (TFV)
Antiviral
Phase I
Not provided
Treatment for people living with HIV
People living with HIV
1 month
Not provided
Lopinavir and ritonavir (LPV/r) , Tenofovir (TFV)
antiviral
Pre-clinical
Not provided
Treatment for people living with HIV to provide sustained viral suppression
People living with HIV
1 month
Not provided
Not provided
Pre-clinical
Not provided
HIV treatment
Adults living with HIV
1 month
Not provided
Publications
Qingxin Mu, Jesse Yu, Lisa A. McConnachie, John C. Kraft, Yu Gao, Gaurav K. Gulati & Rodney J. Y. Ho (2018)
Translation of combination nanodrugs into nanomedicines: lessons learned and future outlook
Journal of Drug Targeting, 26:5-6, 435-447, DOI: 10.1080/1061186X.2017.1419363
The concept of nanomedicine is not new. For instance, some nanocrystals and colloidal drug molecules are marketed that improve pharmacokinetic characteristics of single-agent therapeutics. For the past two decades, the number of research publications on single-agent nanoformulations has grown exponentially. However, formulations advancing to pre-clinical and clinical evaluations that lead to therapeutic products has been limited. Chronic diseases such as cancer and HIV/AIDS require drug combinations, not single agents, for durable therapeutic responses. Therefore, development and clinical translation of drug combination nanoformulations could play a significant role in improving human health. Successful translation of promising concepts into pre-clinical and clinical studies requires early considerations of the physical compatibility, pharmacological synergy, as well as pharmaceutical characteristics (e.g. stability, scalability and pharmacokinetics). With this approach and robust manufacturing processes in place, some drug-combination nanoparticles have progressed to non-human primate and human studies. In this article, we discuss the rationale and role of drug-combination nanoparticles, the pre-clinical and clinical research progress made to date and the key challenges for successful clinical translation. Finally, we offer insight to accelerate clinical translation through leveraging robust nanoplatform technologies to enable implementation of personalised and precision medicine.
John C. Kraft, Lisa A. McConnachie, Josefin Koehn, Loren Kinman, Jianguo Sun, Ann C. Collier, Carol Collins, Danny D. Shen, Rodney J.Y. Ho,
Journal of Controlled Release, Volume 275, 2018, Pages 229-241, ISSN 0168-3659, https://doi.org/10.1016/j.jconrel.2018.02.003.
Existing oral antiretroviral (ARV) agents have been shown in human studies to exhibit limited lymph node penetration and lymphatic drug insufficiency. As lymph nodes are a reservoir of HIV, it is critical to deliver and sustain effective levels of ARV combinations in these tissues. To overcome lymph node drug insufficiency of oral combination ARV therapy (cART), we developed and reported a long-acting and lymphocyte-targeting injectable that contains three ARVs—hydrophobic lopinavir (LPV) and ritonavir (RTV), and hydrophilic tenofovir (TFV)—stabilized by lipid excipients in a nanosuspension. A single subcutaneous (SC) injection of this first-generation formulation of drug combination nanoparticles (DcNPs), named TLC-ART101, provided persistent ARV levels in macaque lymph node mononuclear cells (LNMCs) for at least 1 week, and in peripheral blood mononuclear cells (PBMCs) and plasma for at least 2 weeks, demonstrating long-acting pharmacokinetics for all three drugs. In addition, the lymphocyte-targeting properties of this formulation were demonstrated by the consistently higher intracellular drug concentrations in LNMCs and PBMCs versus those in plasma. To provide insights into the complex mechanisms of absorption and disposition of TLC-ART101, we constructed novel mechanism-based pharmacokinetic (MBPK) models. Based upon plasma PK data obtained after single administration of TLC-ART101 (DcNPs) and a solution formulation of free triple-ARVs, the models feature uptake from the SC injection site that respectively routes free and nanoparticle-associated ARVs via the blood vasculature and lymphatics, and their eventual distribution into and clearance from the systemic circulation. The models provided simultaneous description of the complex long-acting plasma and lymphatic PK profiles for all three ARVs in TLC-ART101. The long-acting PK characteristics of the three drugs in TLC-ART101 were likely due to a combination of mechanisms including: (1) DcNPs undergoing preferential lymphatic uptake from the subcutaneous space, (2) retention in nodes during lymphatic first-pass, (3) subsequent slow release of ARVs into blood circulation, and (4) limited extravasation of DcNP-associated ARVs that resulted in longer persistence in the circulation.
Keywords: Mechanism-based pharmacokinetic modeling; Long-acting; Antiretrovirals; HIV drug combination treatment; Lymphatic targeted drug delivery; Lymphatic drug insufficiency
Mu Q, Yu J, Griffin JI, Wu Y, Zhu L, McConnachie LA, et al. (2020)
PLoSONE 15(3): e0228557.
https://doi.org/10.1371/journal.pone.0228557
Early diagnosis along with new drugs targeted to cancer receptors and immunocheckpoints have improved breast cancer survival. However, full remission remains elusive for metastatic breast cancer due to dose-limiting toxicities of heavily used, highly potent drug combinations such as gemcitabine and paclitaxel. Therefore, novel strategies that lower the effective dose and improve safety margins could enhance the effect of these drug combinations. To this end, we developed and evaluated a novel drug combination of gemcitabine and paclitaxel (GT). Leveraging a simple and scalable drug-combination nanoparticle platform (DcNP), we successfully prepared an injectable GT combination in DcNP (GT DcNP). Compared to a Cremophor EL/ethanol assisted drug suspension in buffer (CrEL), GT DcNP exhibits about 56-fold and 8.6-fold increases in plasma drug exposure (area under the curve, AUC) and apparent half-life of gemcitabine respectively, and a 2.9-fold increase of AUC for paclitaxel. Using 4T1 as a syngeneic model for breast cancer metastasis, we found that a single GT (20/2 mg/kg) dose in DcNP nearly eliminated colonization in the lungs. This effect was not achievable by a CrEL drug combination at a 5-fold higher dose (i.e., 100/10 mg/kg GT). A dose-response study indicates that GT DcNP provided a therapeutic index of ~15.8. Collectively, these data suggest that GT DcNP could be effective against advancing metastatic breast cancer with a margin of safety. As the DcNP formulation is intentionally designed to be simple, scalable, and long-acting, it may be suitable for clinical development to find effective treatment against metastatic breast cancer.
Koehn, Josefina; Iwamoto, Jennifer F.a; Kraft, John C.a; McConnachie, Lisa A.a; Collier, Ann C.b,c; Ho, Rodney J.Y.a,c,d
AIDS: November 13, 2018 - Volume 32 - Issue 17 - p 2463-2467
doi: 10.1097/QAD.0000000000001969
Objective:
To characterize a drug-combination nanoparticle (DcNP) containing water-insoluble lopinavir (LPV) and efavirenz (EFV), and water-soluble tenofovir (TFV), for its potential as a long-acting combination HIV treatment.
Design:
Three HIV drugs (LPV, EFV, TFV) with well established efficacy and safety were coformulated into a single DcNP suspension. Two macaques were administered one subcutaneous injection and drug concentrations in plasma and mononuclear cells (in peripheral blood and lymph nodes) were analyzed over 2 weeks. Pharmacokinetic parameters and cell-to-plasma relationships of LPV, EFV, and TFV were determined.
Results:
This three-in-one nanoformulation provided extended concentrations of all drugs in lymph node cells that were 57- to 228-fold higher than those in plasma. Levels of all three drugs in peripheral blood mononuclear cells persisted for 2 weeks at levels equal to or higher than those in plasma.
Conclusion:
With long-acting characteristics and higher drug penetration/persistence in cells, this three-in-one DcNP may enhance therapeutic efficacy of these well studied HIV drugs due to colocalization and targeting of this three-drug combination to HIV host cells.
Yu Gao, John C. Kraft, Danni Yu, Rodney J.Y. Ho,
Recent developments of nanotherapeutics for targeted and long-acting, combination HIV chemotherapy,
European Journal of Pharmaceutics and Biopharmaceutics,
Volume 138, 2019, Pages 75-91, ISSN 0939-6411,
https://doi.org/10.1016/j.ejpb.2018.04.014.
Combination antiretroviral therapy (cART) given orally has transformed HIV from a terminal illness to a manageable chronic disease. Yet despite the recent development of newer and more potent drugs for cART and suppression of virus in blood to undetectable levels, residual virus remains in tissues. Upon stopping cART, virus rebounds and progresses to AIDS. Current oral cART regimens have several drawbacks including (1) challenges in patient adherence due to pill fatigue or side-effects, (2) the requirement of life-long daily drug intake, and (3) limited penetration and retention in cells within lymph nodes. Appropriately designed injectable nano-drug combinations that are long-acting and retained in HIV susceptible cells within lymph nodes may address these challenges. While a number of nanomaterials have been investigated for delivery of HIV drugs and drug combinations, key challenges involve developing and scaling delivery systems that provide a drug combination targeted to HIV host cells and tissues where residual virus persists. With validation of the drug-insufficiency hypothesis in lymph nodes, progress has been made in the development of drug combination nanoparticles that are long-acting and targeted to lymph nodes and cells. Unique drug combination nanoparticles (DcNPs) composed of three HIV drugs—lopinavir, ritonavir, and tenofovir—have been shown to provide enhanced drug levels in lymph nodes; and elevated drug-combination levels in HIV-host cells in the blood and plasma for two weeks. This review summarizes the progress in the development of nanoparticle-based drug delivery systems for HIV therapy. It discusses how injectable nanocarriers may be designed to enable delivery of drug combinations that are long-lasting and target-selective in physiological contexts (in vivo) to provide safe and effective use. Consistent drug combination exposure in the sites of residual HIV in tissues and cells may overcome drug insufficiency observed in patients on oral cART.
Keywords: Nanomedicine; Long-acting; Targeted; Drug combination; HIV/AIDS
Yu, J., Mu, Q., Perazzolo, S. et al.
Pharm Res 37, 197 (2020). https://doi.org/10.1007/s11095-020-02888-8
Purpose
To develop drug-combination nanoparticles (DcNPs) composed of hydrophilic gemcitabine (G) and hydrophobic paclitaxel (T) and deliver both drugs to metastatic cancer cells.
Methods
GT DcNPs were evaluated based on particle size and drug association efficiency (AE%). The effect of DcNP on GT plasma time-course and tissue distribution was characterized in mice and a pharmacokinetic model was developed. A GT distribution study into cancer nodules (derived from 4 T1 cells) was performed.
Results
An optimized GT DcNP composition (d = 59.2 nm ±9.2 nm) was found to be suitable for IV formulation. Plasma exposure of G and T were enhanced 61-fold and 3.8-fold when given in DcNP form compared to the conventional formulation, respectively. Mechanism based pharmacokinetic modeling and simulation show that both G and T remain highly associated to DcNPs in vivo (G: 98%, T:75%). GT DcNPs have minimal distribution to healthy organs with selective distribution and retention in tumor burdened tissue. Tumor bearing lungs had a 5-fold higher tissue-to-plasma ratio of gemcitabine in GT DcNPs compared to healthy lungs.
Conclusions
DcNPs can deliver hydrophilic G and hydrophobic T together to cancer nodules and produce long acting exposure, likely due to stable GT association to DcNPs in vivo.
Kraft, John C.a; McConnachie, Lisa A.a; Koehn, Josefina; Kinman, Lorena; Collins, Carola; Shen, Danny D.a; Collier, Ann C.b,c; Ho, Rodney J.Y.a,c,d Long-acting combination anti-HIV drug suspension enhances and sustains higher drug levels in lymph node cells than in blood cells and plasma,
AIDS: March 27, 2017 - Volume 31 - Issue 6 - p 765-770
doi: 10.1097/QAD.0000000000001405
Objective:
The aim of the present study was to determine whether a combination of anti-HIV drugs – tenofovir (TFV), lopinavir (LPV) and ritonavir (RTV) – in a lipid-stabilized nanosuspension (called TLC-ART101) could enhance and sustain intracellular drug levels and exposures in lymph node and blood cells above those in plasma.
Design:
Four macaques were given a single dose of TLC-ART101 subcutaneously. Drug concentrations in plasma and mononuclear cells of the blood (PBMCs) and lymph nodes (LNMCs) were analysed using a validated combination LC-MS/MS assay.
Results:
For the two active drugs (TFV, LPV), plasma and PBMC intracellular drug levels persisted for over 2 weeks; PBMC drug exposures were three- to four-fold higher than those in plasma. Apparent terminal half-lives (t1/2) of TFV and LPV were 65.3 and 476.9 h in plasma, and 169.1 and 151.2 h in PBMCs. At 24 and 192 h, TFV and LPV drug levels in LNMCs were up to 79-fold higher than those in PBMCs. Analysis of PBMC intracellular TFV and its active metabolite TFV-diphosphate (TFV-DP) indicated that intracellular exposures of total TFV and TFV-DP were markedly higher and persisted longer than in humans and macaques dosed with oral TFV prodrugs, tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide (TAF).
Conclusions:
A simple, scalable three-drug combination, lipid-stabilized nanosuspension exhibited persistent drug levels in cells of lymph nodes and the blood (HIV host cells) and in plasma. With appropriate dose adjustment, TLC-ART101 may be a useful HIV treatment with a potential to impact residual virus in lymph nodes.
John C. Kraft, Piper M. Treuting & Rodney J. Y. Ho (2018)
Journal of Drug Targeting, 26:5-6, 494-504, DOI: 10.1080/1061186X.2018.1433681
The distributed network of lymph vessels and nodes in the body, with its complex architecture and physiology, presents a major challenge for whole-body lymphatic-targeted drug delivery. To gather physiological and pathological information of the lymphatics, near-infrared (NIR) fluorescence imaging of NIR fluorophores is used in clinical practice due to its tissue-penetrating optical radiation (700–900 nm) that safely provides real-time high-resolution in vivo images. However, indocyanine green (ICG), a common clinical NIR fluorophore, is unstable in aqueous environments and under light exposure, and its poor lymphatic distribution and retention limits its use as a NIR lymphatic tracer. To address this, we investigated in mice the distribution pathways of a novel nanoparticle formulation that stabilises ICG and is optimised for lymphatic drug delivery. From the subcutaneous space, ICG particles provided selective lymphatic uptake, lymph vessel and node retention, and extensive first-pass lymphatic distribution of ICG, enabling 0.2 mm and 5–10 cell resolution of lymph vessels, and high signal-to-background ratios for lymphatic vessel and node networks. Soluble (free) ICG readily dissipated from lymph vessels local to the injection site and absorbed into the blood. These unique characteristics of ICG particles could enable mechanistic studies of the lymphatics and diagnosis of lymphatic abnormalities.
Drug-combination nanoparticles (DcNP) is a nano-formulation of multiple HIV drugs in one injectable. DcNP demonstrated long-acting pharmacokinetics (PK) for all drugs in the blood and lymphatic system of nonhuman primates (NHP). Long-acting is due to stably circulating DcNP and a depot in the lymphatic system during subcutaneous absorption. Because the PK of each drug in DcNP evolves through two species, i.e., drugs that dissociate from DcNP and drugs retained in DcNP (Part 2, presented separately), we describe here a physiologically based PK model of the nanoparticle-free drugs featuring the role of the lymphatic system. The free drug model was built using subcutaneous injections of suspended lopinavir-ritonavir-tenofovir in NHP and validated by external experiments. The model, for the first time, introduces the lymphatic network as part of a whole-body PBPK system and singles out major lymphatic regions: cervical, axillary, hilar, mesenteric, and inguinal nodes. Although the scope of the free-drug modeling was to support the construction of the nanoparticle model (Part 2), such a detailed/regionalized description of the lymphatic system and mononuclear cells represent an unprecedented level of prediction that renders the free drug model extendible to other small-drug molecules targeting the lymphatic system at both the regional and cellular level.
Drug-combination nanoparticles (DcNP) is a nano-formulation of multiple HIV drugs in one injectable. DcNP demonstrated long-acting pharmacokinetics (PK) for all drugs in the blood and lymphatic system of nonhuman primates (NHP). Long-acting is due to stably circulating DcNP and a depot in the lymphatic system during subcutaneous absorption. Because the PK of each drug in DcNP evolves through two species, i.e., drugs that dissociate from DcNP and drugs retained in DcNP (Part 2, presented separately), we describe here a physiologically based PK model of the nanoparticle-free drugs featuring the role of the lymphatic system. The free drug model was built using subcutaneous injections of suspended lopinavir-ritonavir-tenofovir in NHP and validated by external experiments. The model, for the first time, introduces the lymphatic network as part of a whole-body PBPK system and singles out major lymphatic regions: cervical, axillary, hilar, mesenteric, and inguinal nodes. Although the scope of the free-drug modeling was to support the construction of the nanoparticle model (Part 2), such a detailed/regionalized description of the lymphatic system and mononuclear cells represent an unprecedented level of prediction that renders the free drug model extendible to other small-drug molecules targeting the lymphatic system at both the regional and cellular level.
A novel formulation enabled transformation of 3-HIV drugs tenofovir–lamivudine–dolutegravir from short-acting to long-acting all-in-one injectable. Perazzolo, Simonea; Stephen, Zachary R.a; Eguchi, Masaa; Xu, Xiaolina; Delle Fratte, Rachelea; Collier, Ann C.b; Melvin, Ann J.c; Ho, Rodney J.Y.a,d.
AIDS 37(14):p 2131-2136, November 15, 2023. | DOI: 10.1097/QAD.0000000000003706
https://journals.lww.com/aidsonline/abstract/2023/11150/a_novel_formulation_enabled_transformation_of.6.aspx
Objective:
To develop an injectable dosage form of the daily oral HIV drugs, tenofovir (T), lamivudine (L), and dolutegravir (D), creating a single, complete, all-in-one TLD 3-drug-combination that demonstrates long-acting pharmacokinetics.
Design:
Using drug-combination-nanoparticle (DcNP) technology to stabilize multiple HIV drugs, the 3-HIV drugs TLD, with disparate physical-chemical properties, are stabilized and assembled with lipid-excipients to form TLD-in-DcNP. TLD-in-DcNP is verified to be stable and suitable for subcutaneous administration. To characterize the plasma time-courses and PBMC concentrations for all 3 drugs, single subcutaneous injections of TLD-in-DcNP were given to nonhuman primates (NHP, M. nemestrina).
Results:
Following single-dose TLD-in-DcNP, all drugs exhibited long-acting profiles in NHP plasma with levels that persisted for 4 weeks above predicted viral-effective concentrations for TLD in combination. Times-to-peak were within 24 hr in all NHP for all drugs. Compared to a free-soluble TLD, TLD-in-DcNP provided exposure enhancement and extended duration 7.0-, 2.1-, and 20-fold as AUC boost and 10-, 8.3-, and 5.9-fold as half-life extension. Additionally, DcNP may provide more drug exposure in cells than plasma with PBMC-to-plasma drug ratios exceeding one, suggesting cell-targeted drug-combination delivery.
Conclusions:
This study confirms that TLD with disparate properties can be made stable by DcNP to enable TLD concentrations of 4 weeks in NHP. Study results highlighted the potential of TLD-in-DcNP as a convenient all-in-one, complete HIV long-acting product for clinical development.
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing


