Technology name
Extruded Polymer Device (EXPD)
Developer(s)
Type of technology
Polymeric implant
Administration route
Subcutaneous
Development state and regulatory approval
Non-nucleoside reverse transcriptase inhibitors (NNRTI), Unspecified NNRTI
Pre-clinical
Not provided
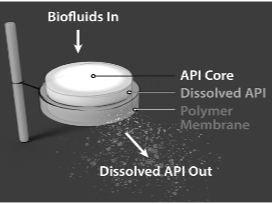
Description
Reservoir implant releasing one or more active pharmaceutical agents with constant release. Implant retrievable during drug release and biodegradable after drug depletion. The implant is 2.5mm x 40 mm. One or two implants can be used simultaneously.
Technology highlight
Bioabsorbable subcutaneous long-acting implant platform with more than 6 month duration; compatible with several potent antiretroviral classes and hormones for HIV and/or contraception; removable for up to at least 6 months (but not required); scalable manufacturing processes.
Illustration(s)
Technology main components
The implant is extruded from a GMP grade polycaprolactone (PCL) used for medical device applications and medical-grade excipients that are generally recognized as safe (GRAS) for subcutaneous administration.
Delivery device(s)
The implant can be delivered through existing, commercially available trocars
APIs compatibility profile
Not provided
Not provided
Not provided
To be provided
2 different APIs : Two or more active pharmaceutical ingredients can be delivered
Not provided
Scale-up and manufacturing prospects
Not provided
Not provided
Not provided
Not provided
Excipients
No proprietary excipient used
No novel excipient or existing excipient used
No residual solvent used
Additional features
- Biodegradable
- Drug-eluting
- Removable
- Single-use
- Reservoir-type
Constant release for at least 6 months.
Not provided
Not provided
Not provided
Not provided
Therapeutic area(s)
- Contraception
- Multipurpose technology : "HIV PrEP and contraception"
- Other(s) : "Breast cancer (treatment and prevention)"
- HIV
- Pre-Exposure Prophylaxis (PrEP)
- Treatment
Potential associated API(s)
- Tenofovir alafenamide (TAF)
- Non-nucleoside reverse transcriptase inhibitors (NNRTI) , Unspecified NNRTI
- Integrase inhibitors (INSTIs)
- Etonogestrel (ENG)
- Selective estrogen receptor modulators (SERMs)
- Islatravir (ISL)
- Levonorgestrel (LNG)
- Islatravir (ISL) , Levonorgestrel (LNG)
Use of technology
- Administered by a nurse
- Administered by a specialty health worker
Every 6 months, Yearly
Not provided
Targeted user groups
- Adults
- All
- Male
- Female
- Cisgender female
- Cisgender male
- Transgender female
- Transgender male
- Intersex
- Gender non-binary
Unspecified
Unspecified
Unspecified
To be further investigated
NRTI ; Antiviral
Pre-clinical
NA
Not provided
Not provided
Not provided
None
Non-nucleoside reverse transcriptase inhibitors (NNRTI) , Unspecified NNRTI
Antiviral
Pre-clinical
Not provided
Not provided
Not provided
Not provided
Not provided
Integrase inhibitors (INSTIs)
INSTIs, Antiretroviral
Pre-clinical
Not provided
Not provided
Not provided
Not provided
Not provided
Etonogestrel (ENG)
Hormones
Pre-clinical
NA
Not provided
Not provided
1 year
None
Selective estrogen receptor modulators (SERMs)
Estrogen receptor
Pre-clinical
Not provided
Not provided
Not provided
Not provided
Not provided
NRTI; Antiviral
Pre-clinical
NA
Not provided
Not provided
6 months
None
Levonorgestrel (LNG)
Hormones
Pre-clinical
NA
Not provided
Not provided
Not provided
None
Islatravir (ISL) , Levonorgestrel (LNG)
antiviral (NRTI) + contraceptive hormone
Pre-clinical
NA
Not provided
Not provided
6 months to 1 year
None
Publications
A critical need exists to develop diverse biomedical strategies for the widespread use of HIV Pre-Exposure Prophylaxis (HIV PrEP). This manuscript describes a subcutaneous reservoir-style implant for long-acting delivery of tenofovir alafenamide (TAF) for HIV PrEP. We detail key parameters of the TAF formulation that affect implant performance, including TAF ionization form, the selection of excipient and the exposure to aqueous conditions. Both in-vitro studies and shelf stability tests demonstrate enhanced performance for TAF freebase (TAFFB) in this long-acting implant platform, as TAFFB maintains higher chemical stability than the TAF hemifumarate salt (TAFHF). We also examined the hydrolytic degradation profiles of various formulations of TAF and identified inflection points for the onset of the accelerated drug hydrolysis within the implant using a two-line model. The compositions of unstable formulations are characterized by liquid chromatography-mass spectrometry (LC-MS) and are correlated to predominant products of the TAF hydrolytic pathways. The hydrolysis rate of TAF is affected by pH and water content in the implant microenvironment. We further demonstrate the ability to substantially delay the degradation of TAF by reducing the rates of drug release and thus lowering the water ingress rate. Using this approach, we achieved sustained release of TAFFB formulations over 240 days and maintained > 93% TAF purity under simulated physiological conditions. The opportunities for optimization of TAF formulations in this biodegradable implant supports further advancement of strategies to address long-acting HIV PrEP.
Keywords: HIV pre-exposure prophylaxis, tenofovir alafenamide, implant, long-acting drug delivery, poly(ε-caprolactone) (PCL), biodegradable polymer
Long-acting (LA) HIV pre-exposure prophylaxis (PrEP) offers the potential to improve adherence by lowering the burden of daily or on-demand regimens of antiretroviral (ARV) drugs. This paper details the fabrication and in vitro performance of a subcutaneous and trocar-compatible implant for the LA delivery of tenofovir alafenamide (TAF). The reservoir-style implant comprises an extruded tube of a biodegradable polymer, poly(ε-caprolactone) (PCL), filled with a formulation of TAF and castor oil excipient. Parameters that affect the daily release rates of TAF are described, including the surface area of the implant, the thickness of the PCL tube walls (between 45 and 200 µm), and the properties of the PCL (e.g., crystallinity). In vitro studies show a linear relationship between daily release rates and surface area, demonstrating a membrane-controlled release mechanism from extruded PCL tubes. Release rates of TAF from the implant are inversely proportional to the wall thickness, with release rates between approximately 0.91 and 0.15 mg/day for 45 and 200 µm, respectively. The sustained release of TAF at 0.28 ± 0.06 mg/day over the course of 180 days in vitro was achieved. Progress in the development of this implant platform addresses the need for new biomedical approaches to the LA delivery of ARV drugs.
Keywords: implant; long-acting drug delivery systems; poly(ε-caprolactone) (PCL); pre-exposure prophylaxis (PrEP); tenofovir alafenamide (TAF).
Introduction: Implants are a new dosage form in development for HIV pre-exposure prophylaxis (PrEP) with potential for high adherence given that they are provider-administered and are intended for long-acting protection. Integrating end-user preference into early stage product development may further overcome challenges with future product uptake and adherence. Hence, we sought to optimize the design of a PrEP implant in early-stage development by gathering opinions about implant attributes from potential end-users in South Africa.
Methods: We conducted 14 focus group discussions (FGDs) with young women and men aged 18 to 24 in Cape Town and Soshanguve, South Africa, inviting participants into discussion as co-designers. FGDs were homogenous by gender and previous implant experience. During FGDs, we showed prototype devices and followed a semi-structured guide with questions on history of contraceptive implant use, preferences for physical characteristics of an implant, implant biodegradability, insertion process, participant-driven ideas for implant design, and social adoption considerations. FGDs were facilitated in English, isiXhosa, Tswana, isiZulu, or Tsonga, audio-recorded, transcribed into English, and qualitatively coded and analysed.
Results: In this qualitative sample of 105 youth (68 women and 37 men), 58 participants were from Soshanguve and 47 from Cape Town, and 23% had previously used contraceptive implants. Participants expressed preferences for several implant design features; specifically, longer duration (≥6 months) was more important to most participants than the size or number of devices implanted. A majority preferred a flexible versus stiff implant to minimize palpability, thereby increasing discreetness and comfort. Nearly all participants favoured a biodegradable implant to avoid removal and thus reduce clinic visits. Concerns about the implant centred on its possible side effects and the "plastic" look of the prototype displayed for demonstration.
Conclusions: This study offers preliminary insights into an implant for HIV prevention that provides long-lasting protection may be well received among young South Africans. Additionally, flexibility, discreetness, and biodegradability may increase acceptability of the implant. Such end-user feedback is being incorporated into current implant designs in the hope of creating an effective long-acting PrEP product that is likely to achieve high uptake and adherence in target populations.
Keywords: South Africa; adolescent; focus groups; implant; pre-exposure prophylaxis; product development.
Implants are in the pre-clinical stage for long-acting HIV pre-exposure prophylaxis (PrEP), with an opportunity to solicit end-users' feedback early in development. Health care providers (HCPs) have been key gatekeepers for contraceptive implant uptake, and uniquely understand both technical considerations and the social context of use. Given their influential role, we gathered South African HCP perspectives on contraceptive implant implementation and features of PrEP implant prototypes that may influence future provider and patient acceptability. We conducted in-depth interviews with 30 HCPs (20 nurses and 10 doctors) in Cape Town and Soshanguve, South Africa. Interviews were conducted by a bioengineer and later transcribed, coded, and analyzed for key themes. HCPs described health system barriers such as understaffed clinics and inadequate training on contraceptive implant removal as major influences to their PrEP implant design preferences. They preferred a PrEP implant that is long lasting (>6 months) to minimize patient-clinic interactions, biodegradable to avoid need for removal, and flexible (but still palpable in case of removal). Commenting on negative experiences with contraceptive implant rollout, they recommended prioritizing both HCP and community education on the PrEP implant, with emphasis on expected side effects, and planning ahead for adequate training of HCPs before rollout. Challenges experienced with past contraceptive implant rollout may taint perspectives on future PrEP implants and must be carefully considered during product development and planning for clinical studies. Particular consideration should be given to the health system context of future distribution, including staff who would be providing and monitoring implants.
Keywords: HIV pre-exposure prophylaxis; South Africa; contraceptive; health care providers; implant; product development.
Local skin reactions were none (grade 0) in 33.3% (LD), 21.2% (MD) and 46.9% (HD) of observations. Mild reactions (grade 1-2) were seen in 61.9% (LD), 69.7% (MD), and 37.5% (HD) of observations. Moderate to severe reactions (grade 3-4) occurred once in the LD group after documented implant breakage and in 9.1% and 3.1% of observations in the MD and HD groups, respectively. H&E staining in the LD and MD group revealed moderate to marked deep dermal inflammation. No local reactions were noted with placebo implants.
Background: Implants promise systemic LA-HIV PrEP delivery while minimizing user burden. Our polycaprolactone (PCL) implant device was re-engineered to a more scalable design that is better aligned with the preferred attributes of a subdermal implant for HIV PrEP as expressed by both medical providers and end users. Here, we assessed the pharmacokinetics and safety of sustained TAF delivery through improved flexible rod-shaped reservoir implants.
Methods: Extruded PCL tubes (40 mm x 2.5 mm) were loaded with a slurry of TAF and castor oil. Ten female rabbits received two implants (one active and one placebo, or two active) subcutaneously inserted via trocar in the dorsal space. Identical TAF implants were concurrently evaluated in vitro. During the 63-d study, rabbits were sampled for plasma TAF/TFV and peripheral blood mononuclear cells (PBMCs) tenofovir-diphosphate (TFV-DP) weekly, and cervical and vaginal tissue TFV-DP on d63.
Results: In vitro TAF release from the implants demonstrated zero-order release kinetics with implants performing as designed, delivering 0.14±0.01 mg/d (low dose) and up to 0.72±0.01 mg/d (high dose). In vivo, PBMC TFV-DP levels of 443±529 fmol/106 and 1293±905 fmol/106 were sustained by the low dose and high doses, respectively. On d63, median vaginal and cervical tissue TFV-DP levels of 139 fmol/mg and 74 fmol/mg were achieved in the high dose group. The plasma TAF/TFV levels were maintained for the study duration. Minimal tissue reactivity at the insertion sites was noted. Residual drug analysis of the retrieved implants indicated similar in vivo and in vitro TAF release rates.
Conclusions: TAF device implants were effectively inserted with an approved trocar, well tolerated, and easily retrieved intact from rabbits after >60 days. All implants delivered TAF and maintained PBMC TFV-DP at putative protective concentrations for the study duration, suggesting our re-designed TAF implant device is suitable for continued development as a long-acting subdermal implant for HIV PrEP.
Li L, Gatto GJ, Brand RM, Krovi SA, Cottrell ML, Norton C, van der Straten A, Johnson LM.
Long-acting biodegradable implant for sustained delivery of antiretrovirals (ARVs) and hormones.
J Control Release. 2021 Oct 20;340:188-199. doi: 10.1016/j.jconrel.2021.10.021. Epub ahead of print. PMID: 34678316.
Women worldwide confront two major reproductive health challenges: the need for contraception and protection from sexually transmitted infections, including Human Immunodeficiency Virus (HIV). Multipurpose Prevention Technologies (MPTs) that simultaneously prevent unintended pregnancy and HIV could address these challenges with a single product. Here, we developed a long-acting (LA) subcutaneously administered and biodegradable implant system that provides sustained delivery of contraceptive and antiretroviral (ARV) with zero-order release kinetics. The MPT system involves two implants comprising an extruded tube of a biodegradable polymer, poly(ε-caprolactone) (PCL). Each implant is filled with a formulation of progestin [levonorgestrel (LNG) or etonogestrel (ENG)], or a formulation of a potent ARV [tenofovir alafenamide (TAF), or 4'-Ethynyl-2-fluoro-2'-deoxyadenosine (EFdA)]. We demonstrated sustained in-vitro release of LNG, ENG, and EFdA from the implant system for 13-17 months, while maintaining high stability of the drugs (>99%) within the implant reservoirs. We further elucidated the controlled release mechanism of the implant and leveraged several tunable parameters (e.g., type and quantity of the excipient, PCL properties, and implant wall thickness) to tailor the release kinetics and enhance the mechanical integrity of the MPT implant. The optimized MPT showed sustained in-vitro release of ENG and EFdA over 1 year while maintaining a high level of formulation stability and structural integrity. The MPT implant system was further evaluated in a preclinical study using a rodent model and demonstrated sustained release of EFdA (6 months) and ENG (12 months) with high stability of the drug formulation (>95%). This manuscript supports the continued advancement of LA delivery systems for MPTs.
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing
Comment & Information
Information related to this technology has been compiled by MPP based on publicly available information. Innovator's review and validation is pending.













