Type of technology
Chemically modified siRNA for self-delivery
Administration route
Intratumoral, Intra-vitreal
Development state and regulatory approval
PH-762
Phase I
FDA has cleared the IND application for PH-762
Description
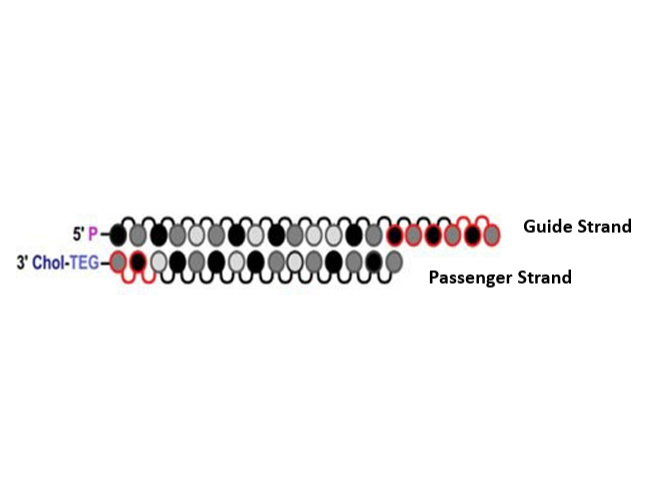
INTASYL™ Technology is a proprietary self-delivering RNA interference (sd-rxRNA®) platform designed for precise gene silencing in immuno-oncology. Unlike traditional RNAi therapies, INTASYL allows direct cellular uptake without requiring complex delivery systems. Engineered for immune cells, it enhances anti-tumor responses while ensuring safety, stability, and efficiency. The sd-rxRNA molecules are chemically modified with hydrophobic and hydrophilic elements, enabling passive diffusion into cells. These modifications also provide stability and resist disintegration.
Developer(s)

Phio Pharmaceuticals, founded in 2011 and headquartered in Marlborough, Massachusetts, is a biotechnology company specializing in RNA-based immuno-oncology therapies. Initially named RXi Pharmaceuticals, it rebranded in 2018 to reflect its focus on self-delivering RNAi technology. The company develops tumor-targeting therapeutics, leveraging cutting-edge RNAi platforms.
Technology highlight
1. Chemically modified siRNA structure and self-delivery system 2. Cellular uptake mechanism via direct membrane penetration 3. Intracellular processing and RNA-induced silencing complex (RISC) Activation 4. Gene silencing and Immuno-oncology applications 5. Target specificity
Illustration(s)
Technology main components
Chemically modified siRNA
Not provided
Delivery device(s)
No delivery device
APIs compatibility profile
INTASYL self-delivery technology utilizes siRNA to specifically target and silence a range of immuno-oncology proteins, including PD-1, BRD4, CTLA4, TIGIT, LAG3, TIM3, CBLB, SHP-1, STAT-3, MDM2, ADORA2, MMP-1, CD96, CISH, CSK, DGKα, DGKζ, DNMT3A, HK2, IL-6, KLRC1, PD-L1, PRDM, PTEN, TBX21, TET2, and other related proteins involved in immune regulation and tumor evasion. Additionally, it targets dermatology-related proteins such as CTGF, COX2, TGFB1, TGFB2, SPP1, TYR, and MMP1, as well as BRD4, contributing to therapeutic strategies in both immuno-oncology and dermatological conditions.
Not provided
Not provided
75-90 wt%
2 different APIs : Two APIs should target different genes simultaneously
Not provided
Scale-up and manufacturing prospects
Not provided
Mermade 12 DNA/RNA Synthesizer
Manufacturing Process of INTASYL (cGMP): 1. Oligonucleotide Synthesis (via Mermade 12 DNA/RNA Synthesizer) 2. Cleavage and Deprotection 3. Purification (via ion exchange chromatography) 4. Sense Strand Purification 5. Desalting and Concentration 6. Analysis by HPLC and ESI-MS.
1. HPLC analysis on a Shimadzu Prominence system. 2. Electrospray Ionization Mass Spectrometry (ESI-MS) analysis using Promass Deconvolution for Xcalibur.
Excipients
No proprietary excipient used
No novel excipient or existing excipient used
No residual solvent used
Additional features
Not provided
When the INSTACYL formulation is administered locally, i.e., intratumorally, the chemically modified siRNA undergoes spontaneous cellular uptake due to the interaction of the lipophilic functional group (e.g., sterol group) with the cell membrane. Thus, the cellular uptake of INSTACYL does not require facilitated transport systems or carriers.
INSTACYL formulation is designed for intratumoral and intravitreal route of adminstration.
The INSTACYL products were well tolerated in the clinical phase 1 studies. One of such study on PH-762 once weekly (for 4 weeks) indicate that there was no No dose-limiting toxicities or clinically significant treatment-emergent adverse effects have been reported.
Not provided
Not provided
Therapeutic area(s)
- Oncology
- Treatment
Potential associated API(s)
- PH-762
- PH-894
Use of technology
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
Weekly
Not provided
Targeted user groups
- Adults
- Older Adults
- All
Unspecified
Unspecified
Unspecified
Not provided
PH-762
PD-1 protein silencer
Phase I
NCT06014086
Cutaneous carcinoma
Not provided
Once weekly
FDA has cleared the IND application for PH-762
PH-894
BRD4 protein silencer
Pre-clinical
Not provided
Melanoma
Not provided
Once weekly
Not provided
Chemically modified oligonucleotides
The disclosure relates, in some aspects, to methods and compositions for production of immunogenic compositions. In some embodiments, the disclosure provides host cells which have been treated ex vivo with one or more oligonucleotide agents capable of controlling and/or reducing the differentiation of the host cell. In some embodiments, compositions and methods described by the disclosure are useful as immunogenic modulators for treating cancer.
AU2018313149B2
Not provided
Phio Pharmaceuticals Corp
Not provided
August 7, 2038
Active
Reduced size self-delivering RNAi compounds
A method for delivering a nucleic acid to a remote target tissue in a subject in need thereof, comprising systemically administering to the subject an sd-rxRNA® in an effective amount to promote RNA interference by the sd-rxRNA® in the remote target tissue, wherein the sd-rxRNA® comprises a guide strand and a passenger strand, wherein the sd-rxRNA® includes a double-stranded region and a single stranded region wherein the double stranded region is from 8-15 nucleotides long, wherein the single stranded region is at the 3′ end of the guide strand and is 4-12 nucleotides long, wherein the single stranded region contains 3, 4, 5, 6, 7, 8, 9, 10, 11, or 12 phosphorothioate modifications, wherein at least 40% of the nucleotides of the sd-rxRNA® are modified, and wherein at least two Us.
US10240149B2
Not provided
Phio Pharmaceuticals Corp
Not provided
March 24, 2031
Active
Phagocytic cell delivery of RNAI
The present invention provides a particulate delivery system for delivering an RNAi construct to phagocytic cells such as macrophages, comprising various configurations of a complex comprising a phagocytic cell-targeting moiety and an RNAi construct. The invention further provides methods of making the delivery system, and their uses, such as treating phagocytic cell-associated disease conditions.
US8815818B2
Not provided
Phio Pharmaceuticals Corp
Not provided
August 16, 2029
Active
Publications
Maxwell, M., Holton, K., Looby, R. J., Byrne, M., & Cardia, J. (2024). Self-Delivering RNAi Compounds for Reduction of Hyperpigmentation. Clinical, cosmetic and investigational dermatology, 17, 3033–3044. https://doi.org/10.2147/CCID.S498987
Purpose: Abnormal melanin synthesis causes hyperpigmentation disorders like melasma and lentigines, impacting psychological well-being. RNA interference (RNAi) uses small RNA molecules to inhibit gene expression by targeting specific mRNA, silencing genes involved in undesirable cellular functions. This study assessed INTASYL compounds, self-delivering RNAi molecules, designed to target and reduce tyrosinase gene expression to decrease pigmentation.
Methods: 36 INTASYL compounds were designed to target and reduce TYR gene expression and tested in a screening assay. RXI-231, the lead compound, was tested in normal human epithelial melanocytes and the MelanoDerm™ model, a 3D reconstituted human epidermal culture. RXI-231 was evaluated for its ability to reduce tyrosinase mRNA expression, in vitro dopachrome formation, and melanin content. Penetration of fluorescently labeled INTASYL compounds through the stratum corneum into the epidermis was tested in cultured porcine skin explants using a DermaPen® microneedle device and a proprietary mixture of penetration enhancers. RXI-231 was also tested for skin irritation in the MatTek EpiDerm™ model to determine its non-irritant profile.
Results: RXI-231 significantly reduced tyrosinase mRNA expression, dopachrome formation, and melanin content in both normal human melanocytes and the MelanoDerm model. Application of INTASYL compounds every other day visibly reduced pigmentation in the 3D epidermal cultures. Penetration studies showed efficient delivery into the epidermis, overcoming the stratum corneum barrier. RXI-231 showed no irritation, with viability above 50% in the MatTek EpiDerm model, confirming its non-irritant profile.
Cuiffo, B., Maxwell, M., Yan, D., Guemiri, R., Boone, A., Bellet, D., Rivest, B., Cardia, J., Robert, C., & Fricker, S. P. (2024). Self-delivering RNAi immunotherapeutic PH-762 silences PD-1 to generate local and abscopal antitumor efficacy. Frontiers in immunology, 15, 1501679. https://doi.org/10.3389/fimmu.2024.1501679
Objective: Immunotherapeutic inhibition of PD-1 by systemically administered monoclonal antibodies is widely used in cancer treatment, but it may cause severe immune-related adverse events (irSAEs). Neoadjuvant PD-1 inhibition before surgery has shown promise in reducing recurrence by stimulating durable antitumor immunity. Local intratumoral (IT) immunotherapy is a potential strategy to minimize irSAEs, but antibodies have limited tumor penetration, making them less suitable for this approach. Therapeutic self-delivering RNAi (INTASYL) is an emerging modality well-suited for neoadjuvant immunotherapy. This study presents preclinical proof-of-concept for PH-762, an INTASYL designed to silence PD-1, currently in clinical development for advanced cutaneous malignancies (ClinicalTrials.gov#NCT06014086).
Methods and analysis: PH-762 pharmacology was characterized in vitro, and in vivo antitumor efficacy was evaluated using a murine analogue (mPH-762) in syngeneic tumor models with varying PD-1 responsiveness. Bilateral Hepa1-6 models assessed abscopal effects of local treatment. Ex vivo analyses explored mechanisms of direct and abscopal efficacy.
Results: PH-762 was rapidly internalized by human T cells, silencing PD-1 mRNA and decreasing PD-1 surface protein, enhancing TCR-stimulated IFN-γ and CXCL10 secretion. In vivo, IT mPH-762 provided robust antitumor efficacy, local and lymphatic biodistribution, and was well tolerated. Ex vivo analyses revealed that IT mPH-762 depleted PD-1 protein, promoted leukocyte and T cell infiltration, and correlated with tumor control. IT mPH-762 also demonstrated efficacy against untreated distal tumors (abscopal effect) by priming systemic antitumor immunity.
Additional documents
No documents were uploaded
Useful links
There are no additional links
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing