Type of technology
Antibody Fragment proteins
Administration route
Intramuscular, Subcutaneous, Intravenous
Development state and regulatory approval
erythropoietin (EPO)
Marketed
Efesa is approved in Indonesia by the Indonesian Food and Drug Adminstration (BPOM)
Description
The novel noncytolytic hybrid Fc (hyFc) is a nonimmunogenic long-acting Fc fusion technology that uses national proteins to maximize drug stability. hyFc acts as a carrier of agonistic protein drugs using naturally existing IgD and IgG4 Fcs without any mutation in the hyFc region. IgD in the hyFC has high hinge flexibility, minimizing protein-to-protein interaction to increase drug efficacy, and lowering cytotoxicity problems through ADCC and CDC. By binding with neonatal Fc receptor (FcRn), IgG4 is recycled (regeneration) in vivo, enabling it to have long-acting pharmacokinetics.
Developer(s)

Genexine is a leading South Korean biopharmaceutical company founded in 1999, focusing on developing and commercializing novel therapies using their two proprietary technologies for unmet medical needs. Their core R&D interest lies in immunotherapies for cancer and next-generation long-acting biologics. Their R&D efforts have yielded positive results.
Technology highlight
• Unmodified Natural Proteins as Carriers • Enhanced Efficacy with Reduced Cytotoxicity • Highly stable and versatile • Broad therapeutic potential • Potentially can be used in combination therapy
Illustration(s)
Technology main components
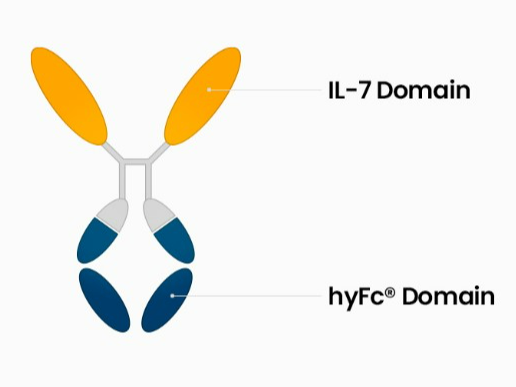
• Core Components: (i) Fc Fusion Protein: This protein is a hybrid of the Fc (fragment crystallizable) region of IgD and IgG4. (ii) IL-7 N-Terminals: These are two separate N-terminals (beginning sections) of the interleukin-7 protein bonded to the API and Fc fusion protein. • Additional Components: (I) Oligopeptide: This is a short chain of amino acids (II) Pharmaceutically accepted adjuvants -buffering agents, dispersing agents (for example: Sodium acetate, sodium chloride, potassium chloride, calcium chloride, Sodium lactate, etc)
Efesa (Long-acting erythropoietin) has been commercially marketed in Indonesia.
Delivery device(s)
No delivery device
APIs compatibility profile
Human growth hormone, bone morphogenetic protein-1 (BMP-1), growth hormone-releasing hormone, growth hormone-releasing peptide, interferons and interferon receptors (e.g., interferon-C, -3, and water-soluble type I interferon receptor, etc.), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), glucagon-like peptides (e.g., GLP-1), G-protein-coupled receptor, interleukins and interleukin receptors, enzymes, interleukin and cytokine binding proteins, macrophage activating factor, monoclonal/polyclonal antibodies are targeted.
Not provided
Not provided
75-90 wt%
2 different APIs : Therapeutic proteins can be combined however further information is not disclosed by the company
Not provided
Scale-up and manufacturing prospects
Efesa (Efepoetin alfa), the inaugural hyFC product, has gained regulatory approval and is commercially accessible in Indonesia. Amid that, Genexine gained a 20,000 square-foot manufacturing facility located in Durham, North Carolina, United States.
Not provided
Manufacturing of Efesa (Efineptakin alfa) in a cleanroom involves: • Construction of a Plasmid Encoding IL 1 Ra-hyFc Fusion Protein • Establishment of Cell Lines Expressing the Present Fusion Protein • Confirmation of Protein Expression by Western • Blotting • Purification and Concentration of Protein • Characterization of hL1 RA-hFC Fusion Protein • Determination and Comparison of Binding Affinity • Detection of IL-8 Using ELISA
• Field emission scanning electron microscope • Transmission electron microscopy • Photon correlation spectroscopy • Enzyme-linked immunosorbent assay (ELISA) • Circular dichroism (CD) spectroscopy • SDS-PAGE and Immunoblotting
Excipients
No proprietary excipient used
No novel excipient or existing excipient used
No residual solvent used
Additional features
- Single-use
- Other(s)
Drug release from the protein fragments at intercellular level
The fusion protein GX-H9, comprising the erythropoietin analog -Efepoietin Alfa and the Fc domain of human IgG4, exhibits a prolonged serum half-life due to FcRn-mediated endocytosis and recycling. The gradual release of Efepoietin Alfa from the IgG4 moiety contributes to this extended pharmacokinetic profile. Following subcutaneous administration, mean serum concentrations of Efepoietin Alfa reached peak levels within 0-12 hours and subsequently declined in a biphasic pattern.
HyFC prefilled injections are administered via subcutaneous or intramuscular injection. The needle is inserted into the designated site, and the medication is injected at a controlled rate
Clinical studies of Efesa Q2W show that 69.7% of the treated anemia in CKD-non-dialysis patients have experienced an AE (vs 64.5%).
hyFC technology products may allow long-term storage at cold storage with a shelf life of 1 year.
Store in a refrigerator at temperatures between 2°C and 8°C. Avoid freezing. Protect from light and handle gently.
Therapeutic area(s)
- Diabetes
- Other(s) : "HPV, Autoimmune diseases, Organ Transplantation, CKD induced anemia, Obesity, Ocular degeneration and Neutropenia"
- Oncology
- Treatment
Potential associated API(s)
- Interleukins
- erythropoietin (EPO)
- Human Growth Hormone Agonists
- Programmed cell death ligand 1 (PD-L1)
- Colony stimulating factors
- Glucagon-like peptide-1 (GLP-1) analogues (GLP-1)
- Recombinant human thyroid-stimulating hormone (rhTSH)
- glucagon-like peptide-2 (GLP-2)
- Interleukins
- Vascular Endothelial Growth Factor inhibitors (VEGF/VEGFR)
- Programmed cell death ligand 1 (PD-L1)
Use of technology
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
Monthly, Every two weeks and Twice monthly
Not provided
Targeted user groups
- Children
- Adolescents
- Adults
- Older Adults
- All
No
No
Unspecified
Not provided
Interleukins
Antineoplastic agent
Phase II
NCT03144934
Lymphopenia, solid tumors, infectious disease
Not provided
Twice monthly
Not provided
erythropoietin (EPO)
Erythropoiesis-stimulating agent
Marketed
NCT06466785
CKG induced Anemia
Chronic kidney diseases patients
Every 2 weeks and 4 weeks
Efesa is approved in Indonesia by the Indonesian Food and Drug Adminstration (BPOM)
Human Growth Hormone Agonists
Pituitary Hormones and analogues
Phase III
Not provided
Pediatric & Adult growth hormone deficiency
Children, adolescents and adults with Growth hormone deficiency
Twice monthly
Eftansomatropin alfa is currently in Phase 3 clinical study in China
Programmed cell death ligand 1 (PD-L1)
PDL1 inhibitors(Programmed death-ligand 1 inhibitors)
Phase I
NCT04298749
Autoimmune disease, and Organ Transplatation
Not provided
Not provided
Not provided
Colony stimulating factors
Granulocyte colony-stimulating factor
Phase I
NCT01951027
Neutropenia
Not provided
Not provided
Not provided
Glucagon-like peptide-1 (GLP-1) analogues (GLP-1)
GLP-1 and its analogues
Phase I
NCT03651466
Diabetes Mellitus and Obesity
Not provided
Not provided
Not provided
Recombinant human thyroid-stimulating hormone (rhTSH)
Recombinant human thyroid stimulating hormone
Phase I
NCT03276988
Differentiated thyroid carcinoma
Patients who are undergoing Total or Partial Thyroidectomy
Not provided
Not provided
glucagon-like peptide-2 (GLP-2)
Glucagon-like peptide-2 (GLP-2) analogs
Phase I
Not provided
Short Bowel Syndrome
Not provided
Not provided
Not provided
Interleukins
Interleukin-7
Phase II
NCT03478995
Fibrotic/metastatic cancers
Not provided
Every three or six weeks
Not provided
Vascular Endothelial Growth Factor inhibitors (VEGF/VEGFR)
VEGR inhibitors
Phase I
Not provided
Neovascular (Wet) Age-Related Macular Degeneration
Not provided
Not provided
Not provided
Programmed cell death ligand 1 (PD-L1)
PD-L1 inhibitors
Phase I
Not provided
Autoimmune disease and Organ Transplantion
Not provided
Not provided
Not provided
Formulation of modified interleukin-7 fusion protein
Provided is a pharmaceutical formulation comprising a modified IL-7 protein. More particularly, it comprises (a) a modified IL-7 fusion protein; (b) a basal buffer with a concentration of 10 to 50 mM; (c) a sugar with a concentration of 2.5 to 5 w/v %; and (d) a surfactant with a concentration of 0.05 to 6 w/v %. Such pharmaceutical formulation of a modified IL-7 fusion protein does not show aggregates formation, but shows protective effects on proteins under stress conditions such as oxidation or agitation, and thus can effectively be used for the treatment of a patient.
US11548927B2
Formulation
Genexine Inc
Not provided
November 2, 2036
Active
Modified interleukin-7 protein
The present invention provides a modified interleukin-7 and a use thereof. The modified IL-7 or an IL-7 fusion protein of the present invention comprising the same can be obtained in high yield, and biologically active in viral infection and cancer models. Therefore, they can be used for the prevention and treatment of various diseases.Genexine Inc
US20190106471A1
Not provided
Genexine Inc
Not provided
October 26, 2036
Active
Human interleukin-1 receptor antagonist—hybrid Fc fusion protein
The present disclosure provides a fusion protein comprising IL-1 receptor antagonist fused to a hybrid Fc. Particularly the present disclosure relates to a fusion protein comprising IL-1 receptor antagonist fused to a human immunoglobulin hybrid Fc fragment. In one embodiment, the hybrid Fc fragment comprises IgD and IgG4. Also provided is a pharmaceutical composition comprising the present fusion protein, which are useful for treating autoimmune disease including rheumatoid arthritis, inflammatory bowel disease (Crohn's disease, ulcerative colitis), psoriasis and diabetes and the like. The present fusion protein with excellent efficacy and reduced side effects is qualified for clinical development as therapeutic antibodies to treat autoimmune disease.
US8883134B2
Formulation
Genexine Co Ltd Handok Pharmaceuticals Co Ltd
Not provided
October 19, 2031
Active
Immunoglobulin fusion proteins
Disclosed are fusion proteins comprising a biologically active molecule and an immunoglobulin (Ig) Fc domain which is linked to the biologically active molecule. The Fc domain is a hybrid human Fc domain of (i) IgG1, IgG2 or IgG4 or (ii) IgG4 and IgD. The hybrid Fc is useful as a carrier of biologically active molecules.
US7867491B2
Treatment
Genexine Co., Ltd.,
Not provided
May 30, 2028
Active
Publications
Kim, Y. J., Koh, E. M., Song, C. H., Byun, M. S., Choi, Y. R., Jeon, E. J., Hwang, K., Kim, S. K., Yang, S. I., & Jung, K. J. (2021). Preclinical immunogenicity testing using anti-drug antibody analysis of GX-G3, Fc-fused recombinant human granulocyte colony-stimulating factor, in rat and monkey models. Scientific reports, 11(1), 12004. https://doi.org/10.1038/s41598-021-91360-7
We optimized and validated analytical tools by adopting validation parameters for immunogenicity assessment. Using these validated tools, we analyzed serum samples from rats and monkeys injected subcutaneously with GX-G3 (1, 3 or 10 mg/kg once a week for 4 weeks followed by a 4-week recovery period) to determine immunogenicity response and toxicokinetic parameters with serum concentration of GX-G3. Several rats and monkeys were determined to be positive for anti-GX-G3 antibodies. Moreover, the immunogenicity response of GX-G3 was lower in monkeys than in rats, which was relevant to show less inhibition of toxicokinetic profiles in monkeys, at least 1 mg/kg administered group, compared to rats. These results suggested the establishment and validation for analyzing anti-GX-G3 antibodies and measurement of serum levels of GX-G3 and anti-GX-G3 antibodies, which were related to toxicokinetic profiles. Taken together, this study provides immunogenicity assessment which is closely implicated with a toxicokinetic study of GX-G3 in 4-week repeated administrated toxicological studies.
Choi, Y. J., Hur, S. Y., Kim, T. J., Hong, S. R., Lee, J. K., Cho, C. H., Park, K. S., Woo, J. W., Sung, Y. C., Suh, Y. S., & Park, J. S. (2020). A Phase II, Prospective, Randomized, Multicenter, Open-Label Study of GX-188E, an HPV DNA Vaccine, in Patients with Cervical Intraepithelial Neoplasia 3. Clinical cancer research : an official journal of the American Association for Cancer Research, 26(7), 1616–1623. https://doi.org/10.1158/1078-0432.CCR-19-1513
We conducted a prospective, randomized, multicenter, open-label, phase II clinical trial of GX-188E in CIN3 patients positive for human papillomavirus (HPV) type 16/18. The primary endpoint was to determine the histopathologic regression to ≤CIN1 at visit seven (V7; 20 weeks after the first GX-188E injection), and an extension study was pursued until visit 8 (V8; 36 weeks after the first GX-188E injection). HPV-sequencing analysis and an ex vivo IFNγ ELISpot assay were performed using the collected cervical biopsy and blood samples from patients.
In total, 72 patients were enrolled and underwent randomization. Of them, 64 patients were included in per-protocol analysis (V7) and 52 in extension analysis (V8). Our data showed 52% (33/64) of patients at V7 and 67% (35/52) of patients at V8 presented histopathologic regression after receiving the GX-188E injection. We found that 73% (V7) and 77% (V8) of the patients with histologic regression showed HPV clearance.
Youn, J. W., Hur, S. Y., Woo, J. W., Kim, Y. M., Lim, M. C., Park, S. Y., Seo, S. S., No, J. H., Kim, B. G., Lee, J. K., Shin, S. J., Kim, K., Chaney, M. F., Choi, Y. J., Suh, Y. S., Park, J. S., & Sung, Y. C. (2020). Pembrolizumab plus GX-188E therapeutic DNA vaccine in patients with HPV-16-positive or HPV-18-positive advanced cervical cancer: interim results of a single-arm, phase 2 trial. The Lancet. Oncology, 21(12), 1653–1660. https://doi.org/10.1016/S1470-2045(20)30486-1
In this open-label, single-arm, phase 2 trial, patients with recurrent or advanced, inoperable cervical cancer, who were aged 18 years or older with Eastern Cooperative Oncology Group performance status of 0 or 1 and histologically confirmed recurrent or advanced HPV-positive (HPV-16 or HPV-18) cervical cancer, and who had progressed after available standard-of-care therapy were recruited from seven hospitals in South Korea. Patients received intramuscular 2 mg GX-188E at weeks 1, 2, 4, 7, 13, and 19, with one optional dose at week 46 that was at the investigator's discretion, and intravenous pembrolizumab 200 mg every 3 weeks for up to 2 years or until disease progression. The primary endpoint was the overall response rate within 24 weeks assessed by the investigator using Response Evaluation Criteria in Solid Tumors version 1.1 in patients who received at least 45 days of treatment 45 days of treatment with at least one post-baseline tumour assessment, and this is the report of a planned interim analysis. This trial is registered with ClinicalTrials.gov, NCT03444376.
Yang, S. H., Yang, S. I., & Chung, Y. K. (2012). A long-acting erythropoietin fused with noncytolytic human Fc for the treatment of anemia. Archives of pharmacal research, 35(5), 757–759. https://doi.org/10.1007/s12272-012-0500-5
The Fc fusion technology has been introduced to generate long-acting antagonistic drugs such as Enbrel, Orencia and Amevive. Here, Genexine created a novel noncytolytic hybrid Fc (hyFc) as a carrier of agonistic protein drugs using naturally existing IgD and IgG4 Fcs without any mutation in the hyFc region. The erythropoietin (EPO) fused with hyFc exhibited little binding activity to FcγR and C1q molecules that are main mediators for death of target cells. The EPO-hyFc showed higher in vitro and in vivo bioactivities than EPOIgG1 Fc and highly glycosylated EPO (Aranesp). Phase I clinical trial with EPO-hyFc is currently undergoing in Korea.
Useful links
There are no additional links
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing
All sponsors
No sponsor indicated