Type of technology
Polymer-based particles, Transdermal patch
Administration route
Subcutaneous, Transdermal
Development state and regulatory approval
Olanzapine
Phase II
US Food and Drug Administration (FDA) has cleared an investigational new drug (IND) application for STAR-OLZ in Chemotherapy in 2022.
Description
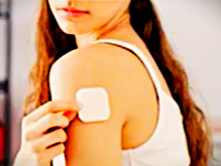
The STARSILON long-acting transdermal patch is a self-administered dosage form that disperses the drug locally in a controlled fashion. This transdermal patch has the potential to modulate pharmacokinetic parameters, including reduced area under the curve (AUC), peak concentration (Cmax), and overall drug exposure. This approach presents opportunities for enhanced therapeutic efficacy and tolerability, as well as the exploration of novel indications or superior outcomes within established treatment areas.
Developer(s)

Starton Therapeutics, a clinical-stage biotechnology enterprise established in 2017, is dedicated to advancing cancer treatment paradigms. The company's core focus is the development of innovative continuous delivery systems to augment existing cancer therapies. This technological approach aims to provide uninterrupted therapeutic exposure for patients with cancer.
Technology highlight
• Self-administered transdermal formulation and injectable options that provide controlled drug release for at least five days • Potential to have a lower incidence of dose-limiting side effects • Elimination of subtherapeutic drug levels due to short half-life APIs
Illustration(s)
Technology main components
• Pressure Sensitive Adhesive (Eg: Styrene - Butadiene - Styrene) • Solubilization Agent / Crystallization Inhibitor (Eg: uncrosslinked polyvinylpyrrolidone ( PVP )) • Thickener (Eg: cassia tora , collagen , gelatin , gellum gum , guar gum , pectin , potassium , or sodium carageenan , tragacanth , xantham , gum copal, chitosan , resin semisynthetic polymers and its derivatives: methylcellulose , ethyl cellulose , carboxymethyl cellulose , hydroxylpropyl cellulose (Klucel HF)) • Skin Permeation Enhancer (Eg: oleic acid , oleyl alcohol ) • Skin Modifiers (Eg: butylated hydroxytoluene; butylated hydroxyanisole) • Polar Aprotic Solvent (Eg: n - methyl - 2 - pyrrolidone (NMP)) • Backing layer (Eg: Scotchpak® 1012) • Release Liner (Eg: Bio - Release® liner) • Penetration enhancers
Raw materials for formulation include polyvinylpyrrolidone (PVP) sourced from BASF Pharma, hydroxypropyl cellulose (Klucel HF) from Ashland, carbomer (Carbopol) from Lubrizol Corporation, colloidal silicon dioxide (AEROSIL) and acrylic polymers (Eudragit) from Evonik Corporation, and polyoxyethylene glycol ether (Brij) from B.J. Bridge. The projected cost of the final product remains undisclosed at this time.
Delivery device(s)
Not provided
APIs compatibility profile
Immunomodulatory agents such as thalodomide, lenalidomide, pomalidomide, iberdomide in combination with dexamethasone, Dronabinol, apremilast analogs , apremilast derivatives , apremilast metabolites , and combinations thereof are targeted for this dosage form.
Not provided
Not provided
Not provided
2 different APIs : Not provided
Not provided
Scale-up and manufacturing prospects
Not provided
• Mixing Apparatus • Laminating Machine • Drying oven • Die-Cutting Machine • Solvent Evaporation System
ISO Class 7 or 8 with HEPA filters. Manufacturing process includes: • Hot melt extrusion process • Pretreatment Composition • Blend Preparation • Coating
• HPLC • Gas Chromatographly (GC) • Differential Scanning Calorimetry (DSC) • Fourier-Transform Infrared Spectroscopy (FTIR) • UV-Visible Spectroscopy • Scanning Electron Microscopy • Tensile Testing Equipment
Excipients
No proprietary excipient used
No novel excipient or existing excipient used
No residual solvent used
Additional features
- Drug-eluting
- Monolithic
- Removable
- Reservoir-type
- Other(s)
Ambulatory Subcutaneous pump
• The release rate of the API from the transdermal patch is influenced by multiple factors, including drug solubility within the matrix, adhesive type, and the incorporation of permeation enhancers. • Preclinical pharmacokinetic studies comparing lenalidomide delivered transdermally to oral administration demonstrated more consistent drug plasma concentration profiles. • This suggests that transdermal delivery may bypass hepatic first-pass metabolism, potentially enhancing the drug's bioavailability.
Not applicable
Interim results from the Phase 1b STAR-LLD trial (2024) demonstrated no hematologic toxicities exceeding Grade 1 after up to three treatment cycles. Additionally, no drug-related non-hematologic toxicities beyond Grade 1 were observed, with only a single instance of Grade 1 dermatologic toxicity reported across four cumulative cycles.
STARSILON transdermal patches offer a versatile drug delivery system, capable of providing sustained release from 24 hours up to 15 days. Stability and shelf life of the patch can be optimized through various pharmaceutical strategies, including co-crystal formation, drug coating, and the incorporation of inert protective agents.
Not provided
Therapeutic area(s)
- Oncology
- Mental health
- Treatment
Potential associated API(s)
- Olanzapine
- lenalidomide
- dronabinol
- Ondansetron
- Thalidomide derivates
Use of technology
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
- Self-administered
Every 5 days
Not provided
Targeted user groups
- Adults
- Older Adults
- All
Unspecified
Unspecified
Unspecified
Not provided
Olanzapine
Antipsychotic
Phase II
Not provided
Chemotherapy-induced nausea and vomiting (CINV)
Not provided
Once every 5 days
US Food and Drug Administration (FDA) has cleared an investigational new drug (IND) application for STAR-OLZ in Chemotherapy in 2022.
lenalidomide
CRL4CRBN E3 ubiquitin ligase modulator
Phase I
Not provided
Multiple Myeloma and Chronic Lymphocytic Leukemia
Not provided
Not provided
A comprehensive data summary for the U.S. Food and Drug Administration (FDA) will be compiled by the company. Additionally, phase II clinical trials are scheduled to commence in 2025.
dronabinol
Antiemetics
Pre-clinical
Not provided
Chemotherapy induced nausea and vomiting
Not provided
Not provided
Not provided
Ondansetron
Antiemetics
Pre-clinical
Not provided
Not provided
Not provided
Not provided
Not provided
Thalidomide derivates
Immunomodulatory Imides
Pre-clinical
Not provided
Not provided
Not provided
Not provided
Not provided
Treatment of Vomiting and Nausea with Minimum Dose of Olanzapine
Compositions, devices, and methods for transdermal administration of olanzapine and uses thereof, e.g., to treat nausea and vomiting.
US20240189251A1
Formulation
Starton Therapeutics, Inc.
Not provided
February 20, 2044
Granted
Transdermal delivery of dronabinol
Provided is a transdermal drug delivery system comprising dronabinol. The dronabinol transdermal delivery system provides a drug plasma concentration at predetermined rate for a predetermined period of time, offering a simplified therapeutic regimen by decreasing dosing frequency for the treatment and/or prevention of nausea and/or vomiting associated with, for example, chemotherapy.
US20210236417A1
Formulation
Starton Therapeutics, Inc.
Not provided
April 7, 2041
Granted
Ondansetron In-adhesive Transdermal Patch
Drug-in-adhesive transdermal patches for the administration of the ondansetron are described. The patches find use, for example, to treat chemotherapy induced nausea and vomiting (CINV), which requires a high initial flux in the acute phase that is sustained over multiple days or weeks. Embodiments of the disclosure relate in part to formulations for manufacturing self-adhesive patches that include various combinations of a variety of advantageous components, which can be tailored to treat various patient groups or symptoms.
WO2020118091A!
Formulation
Starton Therapeutics, Inc.
Not provided
December 5, 2019
Granted
Transdermal drug delivery systems for administration of a therapeutically effective amount of lenalidomide and other immunomodulatory agents
Transdermal drug delivery systems and methods of fabricating such systems are provided. The active pharmaceutical ingredient can be lenalidomide or other immunomodulatory agents. More particularly, the present invention is directed to improving the solubility of lenalidomide and other immunomodulatory imide compounds and improving the permeation of such compounds through the skin.
EP4351536A1
Not provided
Starton Therapeutics, Inc.
Not provided
Not provided
Pending
Publications
There are no publication
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing
All sponsors
No sponsor indicated