Type of technology
Inorganic nanoparticles, Polymer-based particles, Based on other organic particles, Fat based particles, lecithin based particles, Micellar suspension
Administration route
Intravenous, Intratumoral, Subcutaneous
Development state and regulatory approval
Palopegteriparatide
Marketed
YORVIPATH® is approved by the US FDA, EMA, NMPA and Ministry of Food and Drug Safety of South Korea
Description
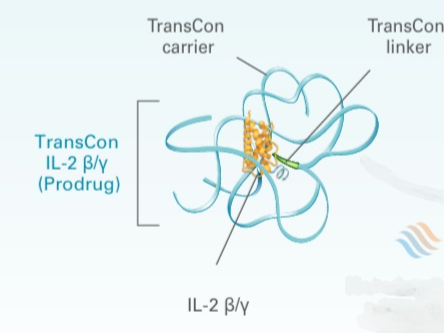
TransCon technology is a carrier-based system that transiently converts an active drug into a prodrug by conjugating it to a carrier via a cleavable linker. This mechanism enables sustained therapeutic drug levels across systemic and localized administration routes. Initially designed for pediatric sustained-release drug delivery, its application now extends to adult diseases, primarily targeting endocrinological disorders and solid tumors. The choice of carrier and linker is tailored to the route of administration and the desired release kinetics of each API.
Developer(s)

Ascendis Pharma A/S, founded in 2006 in Copenhagen, Denmark, develops innovative therapies using its TransCon® technology, focusing on endocrinology and oncology. With a global presence and ~879 employees, its market cap is ~$7.9B (2025). Recent milestones include YORVIPATH® approval for hypoparathyroidism. Despite revenue challenges with SKYTROFA, it remains committed to advancing its pipeline.
Technology highlight
1. TransCon carriers include lipids (ufasomes), polymers (polymerosomes), transfersomes, ethosomes, and sphingosomes. 2. The inertness of the carrier reduces drug clearance, enhancing circulation time. 3. Enables high target-site drug concentrations while minimizing systemic toxicity.
Illustration(s)
Technology main components
1) API—Can be a small molecule, peptide, or protein. 2) Carrier (with Albumin Avidity)—Either soluble or insoluble, tailored to stability and release profile. 3) Reversible prodrug linker—Aromatic Cyclic Imide, DKP Carbamate, Bicin, AEG (2-((2-aminoethyl)amino)acetic acid), Pyroglutamate 4) Functional agents—antiadsorbents, buffering agents, isotonicity modifiers, preservatives, antimicrobials, stabilizers, diffusion enhancers, and oxidation protectants. 5) Excipients—mannitol, metacresol, sodium hydroxide, succinic acid, water for injection, diluents, adjuvants, and vehicles optimized based on the API’s physicochemical properties.
Yorvipath 294 micrograms/0.98ml solution for injection prefilled pen (2 pen pack) costs 9272.97 USD
Delivery device(s)
No delivery device
APIs compatibility profile
TransCon has the ability to encapsulate small molecules, but the targeted spectrum of pharmacological class is not disclosed.
TransCon has the ability to encapsulate RNA molecules, but the targeted spectrum of pharmacological class is not disclosed.
Although TransCon has the ability to conjugate with Antibodies, Antibody Fragments, Proteins, Peptides, currently, TransCon is focused on hormones and cell surface proteins targeting neoplastic cells.
Not provided
Not provided
75-90 wt%
2 different APIs : At least one of the drugs should be a biologically active moiety or a drug that enhances the effect of the other API.
Not provided
Not provided
Scale-up and manufacturing prospects
Novo Nordisk oversees the commercial manufacturing, clinical development, regulatory approval, and market distribution of products within the TransCon Metabolic Disorders pipeline.
Not provided
Not provided
Not provided
Excipients
No proprietary excipient used
No novel excipient or existing excipient used
No residual solvent used
Additional features
- Biodegradable
- Room temperature storage
- At least 1 year shelf life
The unmodified API, encapsulated within the TransCon carrier, is gradually released from the microsphere environment into the surrounding tissues by deconjugating from the linker. This controlled release facilitates the distribution of the API and subsequent activation of target receptors. Additionally, the inactive carrier is dissociated and subsequently eliminated from the system. This mechanism results in pharmacokinetic properties comparable to those observed with daily dosing regimens.
TransCon formulations (pre-filled pen) can be administered via intravenous, subcutaneous, or intratumoral routes. These formulations can be injected using a standard injection kit equipped with a 21–25 gauge needle as a ready-to-use liquid formulation with a pen injector, depending on the designated route of administration and the molecular integrity of the formulation.
In a 52-week efficacy and safety clinical study of TransCon PTH, 72 out of 78 patients (92%) experienced at least one Grade 1 treatment-emergent adverse event (TEAE) by the 52nd week. Additionally, eight patients experienced a serious adverse event (SAE). Treatment-related TEAEs reported in participants included injection site reactions (26%), hypercalcemia (14%), nausea (9%), headache (8%), and hypocalcemia (5%). Two out of 8 SAEs were attributed to hypocalcemia.
TransCon prefilled pens remain stable for at least one year when stored at cold temperatures.
Do not freeze. Store away from heat. Keep TransCon Formulation in the packaging to protect it from light. Until first use, store the formulation in the refrigerator between 2°C to 8°C. After first use, store it for 14 days at room temperature below 30°C (86°F). After each use, remove the needle and put the pen cap on to protect from light. Discard the prefilled pen 14 days after first use.
Therapeutic area(s)
- Other(s) : "Endocrinological disorders"
- Oncology
- Treatment
Potential associated API(s)
- Human Growth Hormone Agonists , Lonasomatropin
- Palopegteriparatide
- CNP agonist
- TLR7/8 agonist
Use of technology
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
Weekly, Monthly
Not provided
Targeted user groups
- Children
- Adolescents
- Adults
- Older Adults
- All
No
Unspecified
Unspecified
Not provided
Human Growth Hormone Agonists , Lonasomatropin
Somatotropins
Marketed
NCT04326374
Pediatric, Adult GHD and Turner Syndrome
Children and adults suffering from Growth Hormone Deficiency
Once weekly
Lonapegsomatropin-tcgd (SKYTROFA®) was approved by USFDA in August 2021 for treating pediatric GHD. It is also approved by EMA, China, Russia, Belarus and South Korea.
Palopegteriparatide
Somatotropin and somatropin agonists
Marketed
NCT04009291
Pediatric and Adult Hypoparathyroidism
Children and adultoscents
Once weekly
YORVIPATH® is approved by the US FDA, EMA, NMPA and Ministry of Food and Drug Safety of South Korea
CNP agonist
Vasodilators
Phase II
NCT04085523
Pediatric Achondroplasia
Children and adolescents
Once weekly
Not provided
TLR7/8 agonist
Immunostimulant - Antineoplastic agent
Phase II
NCT05980598
Solid Tumors
Not provided
Once weekly
Not provided
Combination therapy with controlled-release CNP agonists
The present invention relates to combinations, or pharmaceutical compositions comprising, a CNP agonist, preferably a controlled-release CNP agonist, and at least one further biologically active moiety or drug, and their use in methods for the treatment or prevention of disorders that benefit from stimulating growth, such as achondroplasia.
AU2023201709B2
formulation
Ascendis Pharma Endocrinology Division AS
Not provided
September 28, 2037
Active
Cnp prodrugs with carrier attachment at the ring moiety
The present invention relates to CNP prodrugs in which the carrier is covalently and reversibly attached to the ring moiety of a CNP moiety, to pharmaceutical compositions comprising such CNP prodrugs, to their uses and to methods of treating diseases that can be treated with the CNP prodrugs of the present invention.
AU2023210599B2
Not provided
Ascendis Pharma Endocrinology Division AS
Not provided
May 1, 2037
Active
Publications
Savarirayan, R., Hoernschemeyer, D. G., Ljungberg, M., Zarate, Y. A., Bacino, C. A., Bober, M. B., Legare, J. M., Högler, W., Quattrin, T., Abuzzahab, M. J., Hofman, P. L., White, K. K., Ma, N. S., Schnabel, D., Sousa, S. B., Mao, M., Smith, A., Chakraborty, M., Giwa, A., Winding, B., … McDonnell, C. (2023). Once-weekly TransCon CNP (navepegritide) in children with achondroplasia (ACcomplisH): a phase 2, multicentre, randomised, double-blind, placebo-controlled, dose-escalation trial. EClinicalMedicine, 65, 102258. https://doi.org/10.1016/j.eclinm.2023.102258
Abstract
Background: TransCon CNP (navepegritide) is an investigational prodrug of C-type natriuretic peptide (CNP) designed to allow for continuous CNP exposure with once-weekly dosing. This 52-week phase 2 (ACcomplisH) trial assessed the safety and efficacy of TransCon CNP in children with achondroplasia.
Methods: ACcomplisH is a global, randomised, double-blind, placebo-controlled, dose-escalation trial. Study participants were recruited between June 10, 2020, and September 24, 2021. Eligible participants were prepubertal, aged 2-10 years, with genetically confirmed achondroplasia, and randomised 3:1 to once-weekly subcutaneous injections of TransCon CNP (6, 20, 50, or 100 μg CNP/kg/week) or placebo for 52 weeks. Primary objectives were safety and annualised growth velocity (AGV). ACcomplisH is registered with ClinicalTrials.gov (NCT04085523) and Eudra (CT 2019-002754-22).
Findings: Forty-two participants received TransCon CNP at doses of 6 μg (n = 10; 7 female), 20 μg (n = 11; 3 female), 50 μg (n = 10; 3 female), or 100 μg (n = 11; 6 female) CNP/kg/week, with 15 receiving placebo (5 female). Treatment-emergent adverse events (TEAEs) were mild or moderate with no grade 3/4 events reported. There were 2 serious TEAEs that were assessed as not related to TransCon CNP. Eleven injection site reactions occurred in 8 participants receiving TransCon CNP and no symptomatic hypotension occurred. TransCon CNP demonstrated a dose-dependent improvement in AGV. At 52 weeks, TransCon CNP 100 μg CNP/kg/week significantly improved AGV vs placebo (least squares mean [95% CI] 5.42 [4.74-6.11] vs 4.35 [3.75-4.94] cm/year; p = 0.0218), and improved achondroplasia-specific height SDS from baseline (least squares mean [95% CI] 0.22 [0.02-0·41] vs -0·08 [-0.25 to 0.10]; p = 0.0283). All participants completed the randomised period and continued in the ongoing open-label extension period receiving TransCon CNP 100 μg CNP/kg/week.
Interpretation: This phase 2 trial suggests that TransCon CNP is effective, safe, with low injection site reaction frequency, and may provide a novel, once-weekly treatment option for children with achondroplasia. These results support TransCon CNP at 100 μg CNP/kg/week in the ongoing pivotal trial.
Khan, A. A., Rejnmark, L., Rubin, M., Schwarz, P., Vokes, T., Clarke, B., Ahmed, I., Hofbauer, L., Marcocci, C., Pagotto, U., Palermo, A., Eriksen, E., Brod, M., Markova, D., Smith, A., Pihl, S., Mourya, S., Karpf, D. B., & Shu, A. D. (2022). PaTH Forward: A Randomized, Double-Blind, Placebo-Controlled Phase 2 Trial of TransCon PTH in Adult Hypoparathyroidism. The Journal of clinical endocrinology and metabolism, 107(1), e372–e385. https://doi.org/10.1210/clinem/dgab577
Abstract
Context: Hypoparathyroidism is characterized by insufficient levels of parathyroid hormone (PTH). TransCon PTH is an investigational long-acting prodrug of PTH(1-34) for the treatment of hypoparathyroidism.
Objective: This work aimed to investigate the safety, tolerability, and efficacy of daily TransCon PTH in adults with hypoparathyroidism.
Methods: This phase 2, randomized, double-blind, placebo-controlled 4-week trial with open-label extension enrolled 59 individuals with hypoparathyroidism. Interventions included TransCon PTH 15, 18, or 21 µg PTH(1-34)/day or placebo for 4 weeks, followed by a 22-week extension during which TransCon PTH dose was titrated (6-60 µg PTH[1-34]/day).
Results: By Week 26, 91% of participants treated with TransCon PTH achieved independence from standard of care (SoC, defined as active vitamin D = 0 μg/day and calcium [Ca] ≤ 500 mg/day). Mean 24-hour urine Ca (uCa) decreased from a baseline mean of 415 mg/24h to 178 mg/24h by Week 26 (n = 44) while normal serum Ca (sCa) was maintained and serum phosphate and serum calcium-phosphate product fell within the normal range. By Week 26, mean scores on the generic 36-Item Short Form Health Survey domains increased from below normal at baseline to within the normal range. The Hypoparathyroidism Patient Experience Scale symptom and impact scores improved through 26 weeks. TransCon PTH was well tolerated with no treatment-related serious or severe adverse events.
Conclusion: TransCon PTH enabled independence from oral active vitamin D and reduced Ca supplements (≤ 500 mg/day) for most participants, achieving normal sCa, serum phosphate, uCa, serum calcium-phosphate product, and demonstrating improved health-related quality of life. These results support TransCon PTH as a potential hormone replacement therapy for adults with hypoparathyroidism.
Holten-Andersen, L., Pihl, S., Rasmussen, C. E., Zettler, J., Maitro, G., Baron, J., Heinig, S., Hoffmann, E., Wegge, T., Krusch, M., Faltinger, F., Killian, S., Sprogoe, K., Karpf, D. B., Breinholt, V. M., & Cleemann, F. (2019). Design and Preclinical Development of TransCon PTH, an Investigational Sustained-Release PTH Replacement Therapy for Hypoparathyroidism. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research, 34(11), 2075–2086. https://doi.org/10.1002/jbmr.3824
Abstract
Hypoparathyroidism (HP) is a condition of parathyroid hormone (PTH) deficiency leading to abnormal calcium and phosphate metabolism. The mainstay of therapy consists of vitamin D and calcium supplements, as well as adjunct Natpara (PTH(1-84)). However, neither therapy optimally controls urinary calcium (uCa) or significantly reduces the incidence of hypercalcemia and hypocalcemia. TransCon PTH, a sustained-release prodrug of PTH(1-34) in development for the treatment of HP, was designed to overcome these limitations. To determine the pharmacokinetics and pharmacodynamics of TransCon PTH, single and repeat s.c. dose studies were performed in rats and monkeys. TransCon PTH demonstrated a half-life of 28 and 34 hours in rats and monkeys, respectively. After repeated dosing, an infusion-like profile of the released PTH, characterized by low peak-to-trough levels, was obtained in both species. In intact rats and monkeys, daily subcutaneous administration of TransCon PTH was associated with increases in serum calcium (sCa) levels and decreases in serum phosphate levels (sP). In monkeys, at a single dose of TransCon PTH that increased sCa levels within the normal range, a concurrent decrease in uCa excretion was observed. In 4-week repeat-dose studies in intact rats and monkeys, uCa excretion was comparable to controls across all dose levels despite increases in sCa levels. Further, in a rat model of HP, TransCon PTH normalized sCa and sP levels 24 hours per day. This was in contrast to only transient trends toward normalization of sCa and sP levels with an up to 6-fold higher molar dose of PTH(1-84). After repeated dosing to HP rats, uCa excretion transiently increased, corresponding to increases in sCa above normal range, but at the end of the treatment period, uCa excretion was generally comparable to sham controls. TransCon PTH was well tolerated and the observed pharmacokinetics and pharmacodynamics were in line with the expected action of physiological replacement of PTH.
Additional documents
No documents were uploaded
Useful links
There are no additional links
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing
All sponsors
No sponsor indicated