Type of technology
In-situ forming gel/implant
Administration route
Subcutaneous
Development state and regulatory approval
Cabotegravir (CAB), Medroxyprogesterone acetate (MPA)
Pre-clinical
Not provided
Description
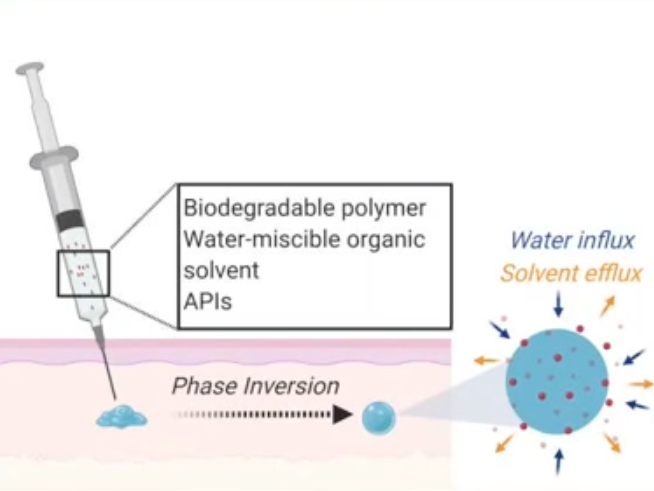
The in-situ forming implant (ISFI) consists of a liquid co-formulation utilizing excipients that form a biodegradable depot after subcutaneous injection for a controlled sustained release of active ingredients. It is based on a biodegradable polymer such as PLGA, a water miscible organic solvent such as NMP and the API or combination APIs of interest.
Technology highlight
First-in-line, ultra-long-acting injectable MPT that offers durable and sustained protection from HIV transmission, high efficacy of contraception, increased user compliance, and the ability to be removed if required. The ISFI offers at least 3 months sustained release after a single subcutaneous administration. It is biodegradable yet removable in case of adverse events or need for treatment reversibility.
Illustration(s)
Technology main components
poly(DL-lactide-co-glycolide) (PLGA), N-methyl-2-pyrrolidone (NMP), dimethyl sulfoxide (DMSO)
Polymers readily available on the market
Delivery device(s)
No delivery device
APIs compatibility profile
Dolutegravir (DTG), Cabotegravir (CAB), Rilpivirine (RPV)
Confidential
Confidential
Not provided
Not provided
To be provided
2 different APIs : at least 2 different APIs
Not provided
Scale-up and manufacturing prospects
To be determined
To be determined
To be determined
To be determined
Excipients
No proprietary excipient used
No novel excipient or existing excipient used
No residual solvent used
Additional features
- Biodegradable
- Drug-eluting
- Monolithic
- Removable
- Single-use
- Room temperature storage
- At least 1 year shelf life
- Needs insertion kit
Zero order release
26-16 gauge needles
Absence of adverse local or systemic inflammation when administered to mice or non-human primates
To be determined
To be determined
Therapeutic area(s)
- Contraception
- Multipurpose technology : "HIV PrEP and contraception"
- Diabetes
- HBV
- TB
- COVID 19
- HIV
- Pain management
- Oncology
- Pre-Exposure Prophylaxis (PrEP)
- Treatment
Potential associated API(s)
- Cabotegravir (CAB) , Medroxyprogesterone acetate (MPA)
- Cabotegravir (CAB) , Etonogestrel (ENG)
- Dolutegravir (DTG) , Medroxyprogesterone acetate (MPA)
- Dolutegravir (DTG) , Etonogestrel (ENG)
Use of technology
- Administered by a nurse
- Administered by a specialty health worker
- To be determined
To be determined
To be determined
Targeted user groups
- Adults
- All
- Male
- Female
- Cisgender female
- Cisgender male
- Transgender female
- Transgender male
- Intersex
- Gender non-binary
Yes
Yes
Unspecified
Not provided
Cabotegravir (CAB) , Medroxyprogesterone acetate (MPA)
Not provided
Pre-clinical
Not provided
Not provided
Not provided
Not provided
Not provided
Cabotegravir (CAB) , Etonogestrel (ENG)
Not provided
Pre-clinical
Not provided
Not provided
Not provided
Not provided
Not provided
Dolutegravir (DTG) , Medroxyprogesterone acetate (MPA)
Not provided
Pre-clinical
Not provided
Not provided
Not provided
Not provided
Not provided
Dolutegravir (DTG) , Etonogestrel (ENG)
Not provided
Pre-clinical
Not provided
Not provided
Not provided
Not provided
Not provided
Publications
Joiner, J. B., King, J. L., Shrivastava, R., Howard, S. A., Cottrell, M. L., Kashuba, A., Dayton, P. A., & Benhabbour, S. R. (2022).
Pharmaceutics, 14(3), 615. https://doi.org/10.3390/pharmaceutics14030615
Due to the versatility of the in situ forming implant (ISFI) drug delivery system, it is crucial to understand the effects of formulation parameters for clinical translation. We utilized ultrasound imaging and pharmacokinetics (PK) in mice to understand the impact of administration route, injection volume, and drug loading on ISFI formation, degradation, and drug release in mice. Placebo ISFIs injected subcutaneously (SQ) with smaller volumes (40 μL) exhibited complete degradation within 30–45 days, compared to larger volumes (80 μL), which completely degraded within 45–60 days. However, all dolutegravir (DTG)-loaded ISFIs along the range of injection volumes tested (20–80 μL) were present at 90 days post-injection, suggesting that DTG can prolong ISFI degradation. Ultrasound imaging showed that intramuscular (IM) ISFIs flattened rapidly post administration compared to SQ, which coincides with the earlier Tmax for drug-loaded IM ISFIs. All mice exhibited DTG plasma concentrations above four times the protein-adjusted 90% inhibitory concentration (PA-IC90) throughout the entire 90 days of the study. ISFI release kinetics best fit to zero order or diffusion-controlled models. When total administered dose was held constant, there was no statistical difference in drug exposure regardless of the route of administration or number of injections.
Keywords: long-acting, in situ, injectable, biodegradable, implants, PLGA, controlled release, drug delivery, ultrasound, pharmacokinetics
Young IC, Benhabbour SR.
Polymers. 2021; 13(15):2450. https://doi.org/10.3390/polym13152450
There is a high global prevalence of HIV, sexually transmitted infections (STIs), and unplanned pregnancies. Current preventative daily oral dosing regimens can be ineffective due to low patient adherence. Sustained release delivery systems in conjunction with multipurpose prevention technologies (MPTs) can reduce high rates of HIV/STIs and unplanned pregnancies in an all-in-one efficacious, acceptable, and easily accessible technology to allow for prolonged release of antivirals and contraceptives. The concept and development of MPTs have greatly progressed over the past decade and demonstrate efficacious technologies that are user-accepted with potentially high adherence. This review gives a comprehensive overview of the latest oral, parenteral, and vaginally delivered MPTs in development as well as drug delivery formulations with the potential to advance as an MPT, and implementation studies regarding MPT user acceptability and adherence. Furthermore, there is a focus on MPT intravaginal rings emphasizing injection molding and hot-melt extrusion manufacturing limitations and emerging fabrication advancements. Lastly, formulation development considerations and limitations are discussed, such as nonhormonal contraceptive considerations, challenges with achieving a stable coformulation of multiple drugs, achieving sustained and controlled drug release, limiting drug–drug interactions, and advancing past preclinical development stages. Despite the challenges in the MPT landscape, these technologies demonstrate the potential to bridge gaps in preventative sexual and reproductive health care.
Additional documents
No documents were uploaded
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing