Drug information
LY03010 (Paliperidone Palmitate Once-Monthly [PP1M])
ERZOFRI®
Small molecule
LY03010 (paliperidone palmitate once-monthly injectable) marketed under the brand name ERZOFRI® is indicated for the treatment of schizophrenia and schizoaffective disorders. A New Drug Application (NDA) was submitted to the U.S. Food and Drug Administration (FDA) based on a multicenter, randomized, open-label relative bioavailability study (NCT04922593) which established the bioequivalence of LY03010 and INVEGA SUSTENNA® at steady state and comparatively evaluated the pharmacokinetics of paliperidone during the initial dosing regimen (IDR). Comparable therapeutic levels of paliperidone were observed during the IDRs of both drugs, however, LY03010 is given consistently once a month from initiation, in comparison to two weekly injections for the initiation doses of INVEGA SUSTENNA®.
A New Drug Application (NDA) for LY03010 was submitted to the U.S. FDA via the 505(b)(2) pathway. Following this submission, Luye Pharma Group Ltd. sent a Paragraph IV patent declaration to the Marketing Authorisation Holder and the patent owner of INVEGA SUSTENNA®. LY03010 was granted a patent (Patent No.11,666,573) in the U.S. in 2023, which will expire in 2039. On July 28 2024, the U.S. FDA approved LY03010 under the brand name ERZOFRI® for the treatment of schizophrenia in adults and schizoaffective disorder in adults as monotherapy, as well as an adjunct to mood stabilizers or antidepressants. Administered once monthly, ERZOFRI® is the first patented paliperidone palmitate long-acting injectable developed in China to be approved in the U.S.
Unknown
Therapeutic area(s)
- Mental health
- Treatment
Administration route
Intramuscular
Associated long-acting platforms
Aqueous drug particle suspension
Use of drug
- Administered by a nurse
- Administered by a specialty health worker
Not provided
Dosage
Not provided
Not provided
Not provided
Not provided
Not provided
Not provided
Formulations
Compare
LY03010 (Paliperidone Palmitate Once-Monthly [PP1M])
Paliperidone Palmitate Once-Monthly (PP1M)
Paliperidone Palmitate Six-Monthly (PP6M)
Paliperidone Palmitate Three Monthly (PP3M)
Associated technologies
Not provided
Comment & Information
Developer(s)

Luye Pharma Group Ltd. is an international pharmaceutical company who develop and manufacture advanced drug delivery systems including microspheres, liposomes and transdermal approaches. The company has established R&D centres in China, the United States and Europe, with a robust pipeline of 40 drug candidates intended for the Chinese market and more than 10 drug candidates overseas.
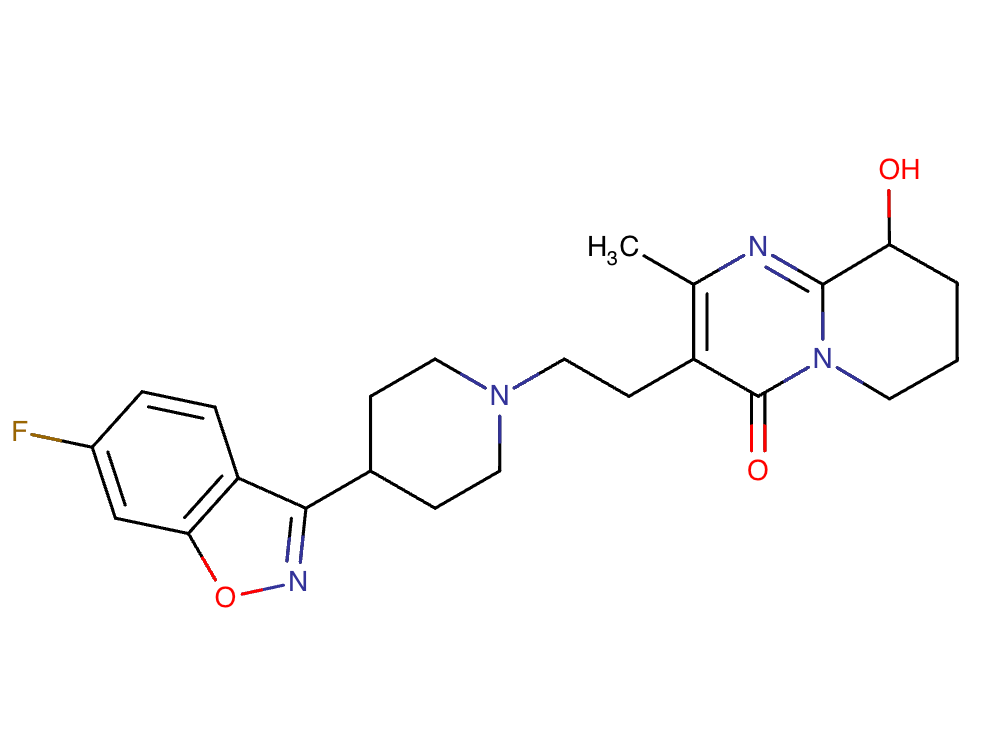
Drug structure
Scale-up and manufacturing prospects
Compound is commercially manufactured. In June 27 2024, Luye Pharma successfully completed a Pre-Approval Inspection (PAI) conducted by the U.S. FDA at its manufacturing facility for LY03010. This inspection is a standard requirement for new drug applications and is designed to assess the facility's compliance with Good Manufacturing Practices (GMP) standards.
Not provided
Not provided
Not provided
Excipients
Not provided
Not provided
Not provided
Delivery device(s)
No delivery device
There are either no relevant patents or these were not yet submitted to LAPaL
Publications
There are no publication
Additional documents
No documents were uploaded
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing