Drug information
Not provided
Paliperidone Palmitate Three-Monthly (PP3M)
INVEGA TRINZA®, TREVICTA® (previously Paliperidone Janssen)
Small molecule
Paliperidone palmitate administered as a once every three-month long-acting injectable (PP3M) is indicated for the maintenance treatment of schizophrenia in patients who have achieved a satisfactory therapeutic response following at least four months of continuous treatment with one-monthly paliperidone palmitate (PP1M) injection. INVEGA TRINZA® and TREVICTA® manufactured by Janssen Pharmaceuticals are available in dosage formulations of 273 mg, 410 mg, 546 mg and 819 mg. Due to its extremely low water solubility, PP3M dissolves gradually following intramuscular injection, prior to being hydrolysed to paliperidone and subsequent absorption. Release of the active paliperidone substance lasts up to 18 months, with maximum plasma concentrations achieved after approx. 30-33 days (median Tmax).
PP3M is approved under the trade name of INVEGA TRINZA® by the United States Food and Drug Administration (US FDA) for the treatment of schizophrenia in patients after they have been adequately treated with INVEGA SUSTENNA® (PP1M) for at least 4 months. PP3M is also approved by the European Medicines Agency (EMA) under the trade name of TREVICTA® (previously Paliperidone Janssen) for the maintenance treatment of schizophrenia in adult patients who are clinically stable on PP1M. Generic formulations of PP3M have not yet been approved in the European Union. PP3M is not currently approved for use in children and adolescents.
Countries where PP3M formulations (INVEGA TRINZA® / TREVICTA®) are approved: Asia Pacific: Australia, Brunei Darussalam, China, Hong Kong, Indonesia, Japan, Macao, Malaysia, New Zealand, Philippines, Singapore, South Korea, Taiwan, Thailand. EMEA: Albania, Bahrain, Bosnia and Herzegovina, Egypt, European Union, Ghana, Israel, Jordan, Kazakhstan, Kenya, Kuwait, Latvia, Lebanon, Montenegro, Morocco, Norway, Oman, Qatar, Russian Federation, Rwanda, Saudi Arabia, Serbia, South Africa, Sudan, Switzerland, Turkey, United Arab Emirates, United Kingdom. Latin America: Argentina, Aruba, Brazil, Chile, Colombia , Costa Rica, Curaçao, Dominican Republic, Ecuador, El Salvador, Guatemala, Honduras, Jamaica, Mexico, Nicaragua, Panama, Peru, Trinidad and Tobago. North America: Canada, USA.
Therapeutic area(s)
- Mental health
- Treatment
Administration route
Intramuscular
Associated long-acting platforms
Nanocrystal technology, Aqueous drug particle suspension
Use of drug
- Administered by a nurse
- Administered by a specialty health worker
Not provided
Dosage
Not provided
Not provided
Not provided
Not provided
Not provided
Not provided
Formulations
Compare
LY03010 (Paliperidone Palmitate Once-Monthly [PP1M])
Paliperidone Palmitate Once-Monthly (PP1M)
Paliperidone Palmitate Six-Monthly (PP6M)
Paliperidone Palmitate Three Monthly (PP3M)
Associated technologies
Not provided
Comment & Information
Developer(s)

Janssen Pharmaceuticals is a subsidiary company of Johnson & Johnson headquartered in Beerse, Belgium. They focus on manufacturing and developing pharmaceutical products for use in areas such as, Immunology, Infectious Diseases & Vaccines, Pulmonary Hypertension, Cardiovascular & Metabolism, Oncology, and Neuroscience.
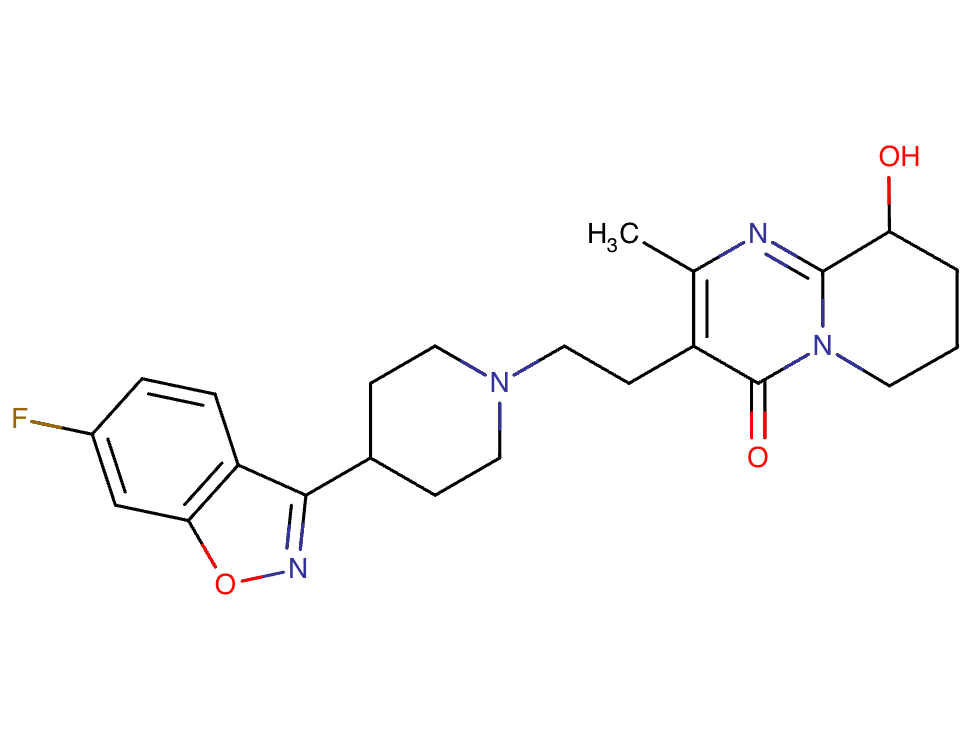
Drug structure
Scale-up and manufacturing prospects
PP3M is commercially manufactured by Janssen Pharmaceuticals with formulation development provided by Alkermes. The PP3M injectable contains a racemic mixture of (+)- and (-)- of paliperidone, which is joined with palmitic acid through an ester linkage. PP3M, like paliperidone palmitate once-monthly injectable (PP1M), is manufactured using Nanocrystal Technology to improve the dissolution properties of paliperidone palmitate, which is highly insoluble in water. PP3M has an extended dosing interval arising from a higher concentration (312 mg/mL) and an increased nanocrystal particle size.
NanoCrystal® Colloidal Dispersion Nanomill™ apparatus.
NanoCrystal technology enables intrinsically high loading of insoluble drugs as dosage forms consist mostly of pure API packed as a solid crystal, which is the most efficient form possible in relation to weight-to-volume. Paliperidone palmitate particles are dispersed in an aqueous suspension and transformed into smaller nanocrystals through particle-size reduction. These nanocrystals have a greater surface area than the larger original particles, resulting in increased water solubility. This medicinal product does not require any special storage conditions and has a shelf life of two years.
Digital microscope and scanning electron microscopy (SEM) to determine shape of the particles. Differential scanning calorimetric (DSC) and Fourier transforms infrared spectroscopy (FTIR) for quality control.
Excipients
No proprietary excipient used
No novel excipient or existing excipient used
No residual solvent used
Delivery device(s)
No delivery device
There are either no relevant patents or these were not yet submitted to LAPaL
Publications
Daghistani N, Rey JA. Invega Trinza: The First Four-Times-a-Year, Long-Acting Injectable Antipsychotic Agent. P T. 2016 Apr;41(4):222-7. PMID: 27069340; PMCID: PMC4811251.
Paliperidone palmitate three-month injection (Invega Trinza): the first four-times-a-year, long-acting injectable antipsychotic agent for schizophrenia.
Lopez A, Rey J. Role of paliperidone palmitate 3-monthly in the management of schizophrenia: insights from clinical practice. Neuropsychiatr Dis Treat. 2019 Feb 11;15:449-456. DOI: 10.2147/NDT.S140383. PMID: 30804673; PMCID: PMC6375110.
Schizophrenia is a complex, chronic psychiatric disorder associated with reduced quality of life and shortened life span. The majority of patients with schizophrenia will relapse within 1 year following an acute episode. The ultimate goals of treatment are to improve functional capabilities, minimize residual symptoms during periods of remission, and decrease relapse frequency and duration, as each relapse brings with it the possibility of a worsening prognosis. Maintaining therapeutic continuity is essential for long-term, positive patient outcomes in schizophrenia. Medication nonadherence and symptomatic relapses magnify the disease burden associated with this disorder. Medication adherence in chronic disease states generally improves with a decrease in dosing frequency. Long-acting injectable (LAI) antipsychotics were developed to improve patient outcomes secondarily to improving medication adherence. Paliperidone palmitate 3-monthly injection (PP3M) is the only LAI available with a quarterly dosing interval. PP3M has been US Food and Drug Administration-approved for use in the long-term maintenance treatment of schizophrenia in patients already controlled on once-monthly PP LAI (paliperidone palmitate once-monthly injection [PP1M]) for a minimum of 4 months. As current evidence supports the efficacy and tolerability of PP3M compared to PP1M and placebo, PP3M appears to be a viable treatment option for patients previously maintained on PP1M. However, to truly establish the place of PP3M in therapy relative to other oral antipsychotics and LAIs, more research is needed. This narrative review aims briefly to describe the pharmacotherapeutic characteristics of PP3M and summarize current literature pertaining to the use of PP3M in the management of schizophrenia.
Additional documents
No documents were uploaded
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing