Drug information
Not provided
Paliperidone Palmitate Once-Monthly (PP1M)
INVEGA SUSTENNA®, XEPLION®, Niapelf
Small molecule
Paliperidone palmitate administered as a once monthly long-acting injectable (PP1M) is indicated for the maintenance treatment of schizophrenia and schizoaffective disorder. INVEGA SUSTENNA® and XEPLION® are manufactured by Janssen Pharmaceuticals and available in dosage strengths of 25mg, 50mg, 100mg, and 150mg. Prior to the initiation of treatment, oral-lead periods to establish tolerability are required for patients naïve to either oral paliperidone or oral or injectable risperidone. Due to its extremely low water solubility, PP1M dissolves slowly following intramuscular injection, prior to being hydrolysed to paliperidone and subsequent absorption. Release of the active paliperidone substance lasts up to 4 months, with maximum plasma concentrations achieved after 13 days (median Tmax).
PP1M has been approved under the trade name of INVEGA SUSTENNA® (Janssen-Cilag Ltd) by the US Food and Drug Administration for the treatment of schizophrenia (approved Aug 2009) & schizoaffective disorder (approved Nov 2015) as monotherapy and as an adjunct to mood stabilisers or antidepressants. The safety and effectiveness of INVEGA SUSTENNA® in patients < 18 years of age have not been established. PP1M is approved by the European Medicines Agency (EMA) under the trade name XEPLION® (Janssen-Cilag Ltd) for the maintenance treatment of schizophrenia in adults whose disease has already been stabilised on treatment with paliperidone or risperidone. The European Commission granted a marketing authorisation valid throughout the European Union for XEPLION® on 4 March 2011.
PP1M is authorised in 102 countries/territories worldwide as of December 6th 2022.
Therapeutic area(s)
- Mental health
- Treatment
Administration route
Intramuscular
Associated long-acting platforms
Aqueous drug particle suspension, Nanocrystal technology
Use of drug
- Administered by a nurse
- Administered by a specialty health worker
Not provided
Dosage
Not provided
Not provided
Not provided
Not provided
Not provided
Not provided
Formulations
Compare
LY03010 (Paliperidone Palmitate Once-Monthly [PP1M])
Paliperidone Palmitate Once-Monthly (PP1M)
Paliperidone Palmitate Six-Monthly (PP6M)
Paliperidone Palmitate Three Monthly (PP3M)
Associated technologies
Not provided
Comment & Information
Developer(s)

Janssen Pharmaceuticals is a subsidiary company of Johnson & Johnson headquartered in Beerse, Belgium. They focus on manufacturing and developing pharmaceutical products for use in areas such as, Immunology, Infectious Diseases & Vaccines, Pulmonary Hypertension, Cardiovascular & Metabolism, Oncology, and Neuroscience.

Neruaxpharm is a European biopharmaceutical company headquartered in both Barcelona, Spain and Langenfeld, Germany. Neuraxpharm specialises in developing medicines and generics for diseases of the central nervous system (CNS). Their portfolio consists of more than 120 molecules for the treatment of Anxiety, Depression, Schizophrenia, Epilepsy, Alzheimer’s, Parkinson’s and other CNS disorders.
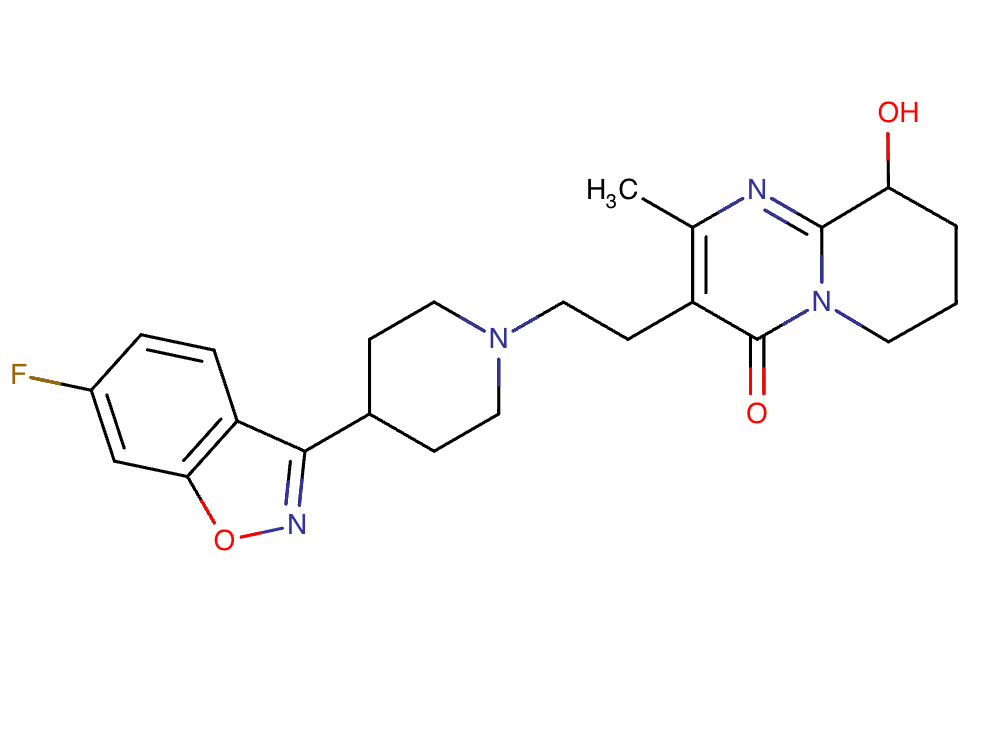
Drug structure
Scale-up and manufacturing prospects
PP1M is commercially manufactured.
NanoCrystal® Colloidal Dispersion Nanomill™ apparatus.
NanoCrystal technology enables intrinsically high loading of insoluble drugs as dosage forms consist mostly of pure API packed as a solid crystal, which is the most efficient form possible in relation to weight-to-volume. Paliperidone palmitate particles are dispersed in an aqueous suspension and transformed into smaller nanocrystals through particle-size reduction. These nanocrystals have a greater surface area than the larger original particles, resulting in increased water solubility. This medicinal product does not require any special storage conditions and has a shelf life of two years.
Digital microscope and scanning electron microscopy (SEM) to determine shape of the particles. Differential scanning calorimetric (DSC) and Fourier transforms infrared spectroscopy (FTIR) for quality control.
Excipients
No proprietary excipient used
No novel excipient or existing excipient used
No residual solvent used
Delivery device(s)
No delivery device
Dosing regimen associated with long acting injectable paliperidone esters
This invention relates to a method of treating patients in need of treatment with long acting injectable paliperidone palmitate formulations
AU2008340101B2
Dosage Regimen
Janssen Pharmaceutica NV
Not provided
December 17, 2028
Active
Publications
Bishara D. Once-monthly paliperidone injection for the treatment of schizophrenia. Neuropsychiatr Dis Treat. 2010 Sep 7;6:561-72. doi: 10.2147/NDT.S8505. PMID: 20856919; PMCID: PMC2938305.
Paliperidone palmitate is a new long-acting antipsychotic injection for the treatment of acute and maintenance therapy in schizophrenia. Paliperidone (9-hydroxyrisperidone) is the major active metabolite of risperidone and acts at dopamine D2 and serotonin 5HT2A receptors. As with other atypical antipsychotics, it exhibits a high 5HT2A:D2 affinity ratio. It also has binding activity as an antagonist at α1-and α2 adrenergic receptors and H1 histaminergic receptors, but has virtually no affinity for cholinergic receptors. Paliperidone palmitate has been shown to be effective in reducing Positive and Negative Syndrome Scale total scores in four short-term trials in acute schizophrenia. It was also effective as maintenance therapy in a long-term trial in which time to recurrence of symptoms was significantly longer in paliperidone-treated patients compared with placebo. In addition, paliperidone was shown to be noninferior to risperidone long-acting injection in one study, but this noninferiority was not established in another longer study comparing the two drugs. Treatment should be initiated with 234 mg on day 1 and 156 mg on day 8, followed by a recommended monthly maintenance dose of 39–234 mg based on efficacy and tolerability. Paliperidone palmitate is generally well tolerated, although it can cause weight gain and a rise in prolactin levels, which is generally greater in women than in men. Overall, paliperidone palmitate may have advantages over other currently available long-acting injections, and therefore may be a useful alternative for the treatment of schizophrenia, although further long-term trials comparing it with active treatments are warranted.
Additional documents
No documents were uploaded
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing